Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels
- PMID: 16607019
- PMCID: PMC1459661
- DOI: 10.1128/EC.5.4.723-731.2006
Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels
Abstract
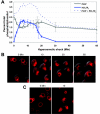
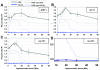
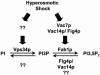
Phosphoinositide lipids regulate complex events via the recruitment of proteins to a specialized region of the membrane at a specific time. Precise control of both the synthesis and turnover of phosphoinositide lipids is integral to membrane trafficking, signal transduction, and cytoskeletal rearrangements. Little is known about the acute regulation of the levels of these signaling lipids. When Saccharomyces cerevisiae cells are treated with hyperosmotic medium the levels of phosphatidylinositol 3,5-bisphosphate (PI3,5P(2)) increase 20-fold. Here we show that this 20-fold increase is rapid and occurs within 5 min. Surprisingly, these elevated levels are transient. Fifteen minutes following hyperosmotic shock they decrease at a rapid rate, even though the cells remain in hyperosmotic medium. In parallel with the rapid increase in the levels of PI3,5P(2), vacuole volume decreases rapidly. Furthermore, concomitant with a return to basal levels of PI3,5P(2) vacuole volume is restored. We show that Fig 4p, consistent with its proposed role as a PI3,5P(2) 5-phosphatase, is required in vivo for this rapid return to basal levels of PI3,5P(2). Surprisingly, we find that Fig 4p is also required for the hyperosmotic shock-induced increase in PI3,5P(2) levels. These findings demonstrate that following hyperosmotic shock, large, transient changes occur in the levels of PI3,5P(2) and further suggest that Fig 4p is important in regulating both the acute rise and subsequent fall in PI3,5P(2) levels.
Figures



References
-
- Audhya, A., and S. D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2:593-605. - PubMed
-
- Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

