The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress
- PMID: 16607396
- PMCID: PMC1456887
- DOI: 10.1038/sj.embor.7400645
The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress
Abstract
Glutathione is a ubiquitous molecule found in all parts of the cell where it fulfils a range of functions from detoxification to protection from oxidative damage. It provides the main redox buffer for cells and as such has been implicated in the formation of native disulphide bonds. However, the discovery of the enzyme Ero1 has called into question the exact role of glutathione in this process. In this review, we discuss the arguments for and against a role for glutathione in facilitating disulphide-bond formation and consider its role in protecting the cell from endoplasmic-reticulum-generated oxidative stress.
Figures

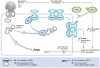

References
-
- Banhegyi G, Lusini L, Puskas F, Rossi R, Fulceri R, Braun L, Mile V, di Simplicio P, Mandl J, Benedetti A (1999) Preferential transport of glutathione versus glutathione disulfide in rat liver microsomal vesicles. J Biol Chem 274: 12213–12216 - PubMed
-
- Bass R, Ruddock LW, Klappa P, Freedman RB (2004) A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem 279: 5257–5262 - PubMed
-
- Chakravarthi S, Bulleid NJ (2004) Glutathione is required to regulate the formation of native disulphide bonds within proteins entering the secretory pathway. J Biol Chem 279: 39872–39879 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

