Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development
- PMID: 16607638
- PMCID: PMC3869087
- DOI: 10.1002/dvdy.20821
Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development
Abstract
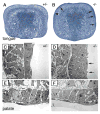
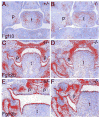
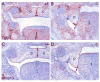
During mammalian palatogenesis, palatal shelves initially grow vertically from the medial sides of the paired maxillary processes flanking the developing tongue and subsequently elevate and fuse with each other above the tongue to form the intact secondary palate. Pathological palate-mandible or palate-tongue fusions have been reported in humans and other mammals, but the molecular and cellular mechanisms that prevent such aberrant adhesions during normal palate development are unknown. We previously reported that mice deficient in Jag2, which encodes a cell surface ligand for the Notch family receptors, have cleft palate associated with palate-tongue fusions. In this report, we show that Jag2 is expressed throughout the oral epithelium and is required for Notch1 activation during oral epithelial differentiation. We show that Notch1 is normally highly activated in the differentiating oral periderm cells covering the developing tongue and the lateral oral surfaces of the mandibular and maxillary processes during palate development. Oral periderm activation of Notch1 is significantly attenuated during palate development in the Jag2 mutants. Further molecular and ultrastructural analyses indicate that oral epithelial organization and periderm differentiation are disrupted in the Jag2 mutants. Moreover, we show that the Jag2 mutant tongue fused to wild-type palatal shelves in recombinant explant cultures. These data indicate that Jag2-Notch1 signaling is spatiotemporally regulated in the oral epithelia during palate development to prevent premature palatal shelf adhesion to other oral tissues and to facilitate normal adhesion between the elevated palatal shelves.
(c) 2006 Wiley-Liss, Inc.
Figures

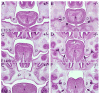
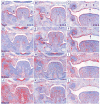
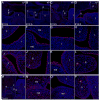
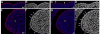



References
-
- Akiyama M, Smith LT, Yoneda K, Holbrook KA, Hohl D, Shimizu H. Periderm cells form cornified cell envelope in their regression process during human epidermal development. J Invest Dermatol. 1999;112:903–909. - PubMed
-
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen Y. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol. 2005;277:102–113. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

