Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer
- PMID: 16609087
- PMCID: PMC1459540
- DOI: 10.1001/jama.295.14.1658
Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer
Erratum in
- JAMA. 2006 May 24;295(20):2356
Abstract
Context: Breast cancer estrogen-receptor (ER) status is useful in predicting benefit from endocrine therapy. It may also help predict which patients benefit from advances in adjuvant chemotherapy.
Objective: To compare differences in benefits from adjuvant chemotherapy achieved by patients with ER-negative vs ER-positive tumors.
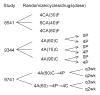
Design, setting, and patients: Trial data from the Cancer and Leukemia Group B and US Breast Cancer Intergroup analyzed; patient outcomes by ER status compared using hazards over time and multivariate models. Randomized trials comparing (1): 3 regimens of cyclophosphamide, doxorubicin, and fluorouracil (January 1985 to April 1991); (2) 3 doses of doxorubicin concurrent with cyclophosphamide, with or without subsequent paclitaxel (May 1994 to April 1997); (3) sequential doxorubicin, paclitaxel, and cyclophosphamide with concurrent doxorubicin and cyclophosphamide followed by paclitaxel, and also 3-week vs 2-week cycles (September 1997 to March 1999). A total of 6644 node-positive breast cancer patients received adjuvant treatment.
Main outcome measures: Disease-free and overall survival.
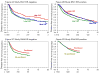
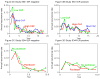
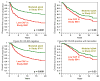
Results: For ER-negative tumors, chemotherapy improvements reduced the relative risk of recurrence by 21%, 25%, and 23% in the 3 studies, respectively, and 55% comparing the lowest dose in the first study with biweekly cycles in the third study. Corresponding relative risk reductions for ER-positive tumors treated with tamoxifen were 9%, 12%, and 8% in the 3 studies, and 26% overall. The overall mortality rate reductions associated with chemotherapy improvements were 55% and 23% among ER-negative and ER-positive patients, respectively. All individual ER-negative comparisons and no ER-positive comparisons were statistically significant. Absolute benefits due to chemotherapy were greater for patients with ER-negative compared with ER-positive tumors: 22.8% more ER-negative patients survived to 5 years disease-free if receiving chemotherapy vs 7.0% for ER-positive patients; corresponding improvements for overall survival were 16.7% vs 4.0%.
Conclusion: Among patients with node-positive tumors, ER-negative breast cancer, biweekly doxorubicin/cyclophosphamide plus paclitaxel lowers the rate of recurrence and death by more than 50% in comparison with low-dose cyclophosphamide, doxorubicin, and fluorouracil as used in the first study.
Figures



References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. - PubMed
-
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. - PubMed
-
- Coombes RC, Hall E, Gibson LJ, et al. for the Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. - PubMed
-
- International Breast Cancer Study Group (IBCSG) Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2002;94(14):1054–1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

