Influence of gastric inhibitory polypeptide on pentagastrin-stimulated gastric acid secretion in patients with type 2 diabetes and healthy controls
- PMID: 16609993
- PMCID: PMC4087512
- DOI: 10.3748/wjg.v12.i12.1874
Influence of gastric inhibitory polypeptide on pentagastrin-stimulated gastric acid secretion in patients with type 2 diabetes and healthy controls
Abstract
Aim: Gastric inhibitory polypeptide is secreted from intestinal K-cells in response to nutrient ingestion and acts as an incretin hormone in human physiology. While animal experiments suggested a role for GIP as an inhibitor of gastric secretion, the GIP effects on gastric acid output in humans are still controversial.
Methods: Pentagastrin was administered at an infusion rate of 1 microg . kg(-1) . h(-1) over 300 min in 8 patients with type 2 diabetes (2 female, 6 male, 54+/- 10 years, BMI 30.5+/- 2.2 kg/m(2); no history of autonomic neuropathy) and 8 healthy subjects (2/6, 46+/- 6 years., 28.9+/- 5.3 kg/m(2)). A hyperglycaemic clamp (140 mg/dl) was performed over 240 min. Placebo, GIP at a physiological dose (1 pmol . kg(-1) . min(-1)), and GIP at a pharmacological dose (4 pmol . kg(-1) . min(-1)) were administered over 60 min each. Boluses of placebo, 20 pmol GIP/kg, and 80 pmol GIP/kg were injected intravenously at the beginning of each infusion period, respectively. Gastric volume, acid and chloride output were analysed in 15-min intervals. Capillary and venous blood samples were drawn for the determination of glucose and total GIP. Statistics were carried out by repeated-measures ANOVA and one-way ANOVA.
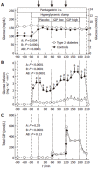
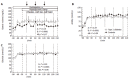
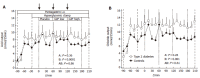
Results: Plasma glucose concentrations during the hyperglycaemic clamp experiments were not different between patients with type 2 diabetes and controls. Steady-state GIP plasma levels were 61+/- 8 and 79+/- 12 pmol/l during the low-dose and 327+/- 35 and 327+/- 17 pmol/l during the high-dose infusion of GIP, in healthy control subjects and in patients with type 2 diabetes, respectively (P=0.23 and P=0.99). Pentagastrin markedly increased gastric acid and chloride secretion (P< 0.001). There were no significant differences in the rates of gastric acid or chloride output between the experimental periods with placebo or any dose of GIP. The temporal patterns of gastric acid and chloride secretion were similar in patients with type 2 diabetes and healthy controls (P=0.86 and P=0.61, respectively).
Conclusion: Pentagastrin-stimulated gastric acid secretion is similar in patients with type 2 diabetes and healthy controls. GIP administration does not influence gastric acid secretion at physiological or pharmacological plasma levels. Therefore, GIP appears to act as an incretin rather than as an enterogastrone in human physiology.
Figures
Similar articles
-
Secretion of incretin hormones and the insulinotropic effect of gastric inhibitory polypeptide in women with a history of gestational diabetes.Diabetologia. 2005 Sep;48(9):1872-81. doi: 10.1007/s00125-005-1863-7. Epub 2005 Jul 12. Diabetologia. 2005. PMID: 16010522
-
Lack of effect of synthetic human gastric inhibitory polypeptide and glucagon-like peptide 1 [7-36 amide] infused at near-physiological concentrations on pentagastrin-stimulated gastric acid secretion in normal human subjects.Digestion. 1992;52(3-4):214-21. doi: 10.1159/000200956. Digestion. 1992. PMID: 1459356 Clinical Trial.
-
Stimulation of insulin secretion by intravenous bolus injection and continuous infusion of gastric inhibitory polypeptide in patients with type 2 diabetes and healthy control subjects.Diabetes. 2004 Dec;53 Suppl 3:S220-4. doi: 10.2337/diabetes.53.suppl_3.s220. Diabetes. 2004. PMID: 15561915
-
Glucose-dependent insulinotropic polypeptide: effects on insulin and glucagon secretion in humans.Dan Med J. 2016 Apr;63(4):B5230. Dan Med J. 2016. PMID: 27034187 Review.
-
No evidence of tachyphylaxis for insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) in subjects with type 2 diabetes, their first-degree relatives, or in healthy subjects.Peptides. 2020 Mar;125:170176. doi: 10.1016/j.peptides.2019.170176. Epub 2019 Oct 25. Peptides. 2020. PMID: 31669136 Review.
References
-
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. - PubMed
-
- Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry. 1978;56:37–44. - PubMed
-
- Krarup T. Immunoreactive gastric inhibitory polypeptide. Endocr Rev. 1988;9:122–133. - PubMed
-
- Meier JJ, Nauck MA. Clinical endocrinology and metabolism. Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide. Best Pract Res Clin Endocrinol Metab. 2004;18:587–606. - PubMed
-
- Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–917. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

