Signal transduction of bombesin-induced circular smooth muscle cell contraction in cat esophagus
- PMID: 16610033
- PMCID: PMC4087658
- DOI: 10.3748/wjg.v12.i14.2259
Signal transduction of bombesin-induced circular smooth muscle cell contraction in cat esophagus
Abstract
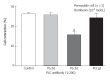
Aim: To investigate the mechanism of bombesin-induced circular smooth muscle cell contraction in cat esophagus.
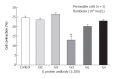
Methods: Specific G protein or phospholipase C involved in cat esophagus contraction was identified, muscle cells were permeabilized with saponin. After permeabilization of muscle cells, the Gi3 antibody inhibited bombesin-induced smooth muscle cell contraction.
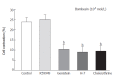
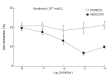
Results: Incubation of permeabilized circular muscle cells with PLC-beta3 antibody could inhibit bombesin-induced contraction. H-7, chelerythrine (PKC inhibitor) and genistein (protein tyrosine kinase inhibitor) inhibited bombesin-induced contraction, but DAG kinase inhibitor, R59949, could not inhibit it. To examine which mitogen-activated protein kinase (MAPK) was involved in bombesin-induced contraction, the specific MAPK inhibitors (MEK inhibitor, PD98059 and p38 MAPK inhibitor, SB202190) were used. Preincubation of PD98059 blocked the contraction induced by bombesin in a concentration-dependent manner. However, SB202190 had no effects on contraction.
Conclusion: Bombesin-induced circular muscle cell contraction in cat esophagus is madiated via a PKC or a PTK-dependent pathway or p44/p42 MAPK pathway.
Figures
References
-
- Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27:166–167. - PubMed
-
- Ganter MT, Pittet JF. Bombesin-like peptides: modulators of inflammation in acute lung injury. Am J Respir Crit Care Med. 2006;173:1–2. - PubMed
-
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268:5979–5984. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

