Therapy for the Brugada syndrome
- PMID: 16610350
- PMCID: PMC1474239
- DOI: 10.1007/3-540-29715-4_12
Therapy for the Brugada syndrome
Abstract
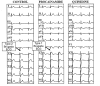
The Brugada syndrome is a congenital syndrome of sudden cardiac death first described as a new clinical entity in 1992. Electrocardiographically characterized by a distinct coved-type ST segment elevation in the right precordial leads, the syndrome is associated with a high risk for sudden cardiac death in young and otherwise healthy adults, and less frequently in infants and children. The ECG manifestations of the Brugada syndrome are often dynamic or concealed and may be revealed or modulated by sodium channel blockers. The syndrome may also be unmasked or precipitated by a febrile state, vagotonic agents, alpha-adrenergic agonists, beta-adrenergic blockers, tricyclic or tetracyclic antidepressants, a combination of glucose and insulin, and hypokalemia, as well as by alcohol and cocaine toxicity. An implantable cardioverter-defibrillator (ICD) is the most widely accepted approach to therapy. Pharmacological therapy aimed at rebalancing the currents active during phase 1 of the right ventricular action potential is used to abort electrical storms, as an adjunct to device therapy, and as an alternative to device therapy when use of an ICD is not possible. Isoproterenol and cilostazol boost calcium channel current, and drugs like quinidine inhibit the transient outward current, acting to diminish the action potential notch and thus suppress the substrate and trigger for ventricular tachycardia/fibrillation (VT/VF).
Figures








References
-
- Aligs M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. - PubMed
-
- Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. - PubMed
-
- Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. f Cardiovasc Electrophysiol. 2001a;12:268–272. - PubMed
-
- Antzelevitch C. The Brugada syndrome. Diagnostic criteria and cellular mechanisms. Eur Heart J. 2001b;22:356–363. - PubMed
-
- Antzelevitch C, Brugada R. Fever and the Brugada syndrome. Pacing Clin Electrophysiol. 2002;25:1537–1539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

