Diffusion of lipid-like single-molecule fluorophores in the cell membrane
- PMID: 16610918
- PMCID: PMC1702323
- DOI: 10.1021/jp0574145
Diffusion of lipid-like single-molecule fluorophores in the cell membrane
Abstract
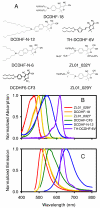
The dicyanomethylenedihydrofuran (DCDHF) class of single-molecule fluorophores contains an amine donor and a dicyanomethylenedihydrofuran acceptor linked by a conjugated unit (benzene, naphthalene, or styrene). Molecules in this class have a number of useful properties in addition to those usually required for single-molecule studies (such as high fluorescence quantum yield and photostability), including second-order optical nonlinearity, large ground-state dipole moment, and sensitivity to local environment. Moreover, most DCDHF molecules have amphiphilic structures, with a polar dicyanomethylenedihydrofuran headgroup and nonpolar hydrocarbon tails on the amine or furan ring, and can be used as fluorescent lipid analogues for live cell imaging. Here we demonstrate that individual molecules of several different DCDHF lipid analogues can be observed diffusing in the plasma membrane of Chinese hamster ovary cells. The photophysical and diffusive behaviors of the DCDHF lipid analogues in membranes are described and are found to be competitive with the well-known lipid probe N-(6-tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine.
Figures




References
-
- Brown DA, London E. Ann. Rev. Cell & Dev. Bio. 1998;14:111–136. - PubMed
-
- Anderson RGW, Jacobson K. Science. 2002;296:1821–1825. - PubMed
-
- Edidin M. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257–283. - PubMed
-
- Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Annual Review of Physical Chemistry. 2005;56:309–336. - PubMed
-
- Schmidt T, Schutz GJ, Baumgartner W, Gruber HJ, Schindler H. J. Phys. Chem. 1995;99:17662–17668.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

