Current strategies and future directions for eluding adenoviral vector immunity
- PMID: 16611043
- PMCID: PMC1455550
- DOI: 10.2174/156652306776359478
Current strategies and future directions for eluding adenoviral vector immunity
Abstract
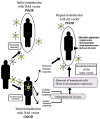
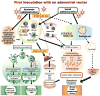
Adenoviral (Ad) vectors can efficiently transduce a broad range of cell types and have been used extensively in preclinical and clinical studies for gene delivery applications. The presence of preexisting Ad immunity in the majority of human population and a rapid development of immune response against the Ad vector backbone following the first inoculation with the vector have impeded clinical use of these vectors. In addition, a number of animal inoculation studies have demonstrated that high systemic doses of Ad vectors invariably lead to initiation of acute inflammatory responses. This is mainly due to activation of innate immunity by vector particles. In general, vector and innate immune responses drastically limit the vector transduction efficiency and the duration of transgene expression. In order to have a predictable response with Ad vectors for gene therapy applications, the above limitations must be overcome. Strategies that are being examined to circumvent these drawbacks of Ad vectors include immunosuppression, immunomodulation, serotype switching, use of targeted Ad vectors, microencapsulation of Ad vectors, use of helper-dependent (HD) Ad vectors, and development of nonhuman Ad vectors. Here we review the current understanding of immune responses to Ad vectors, and recent advances in the strategies for immune evasion to improve the vector transduction efficiency and the duration of transgene expression. Development of novel strategies for targeting specific cell types would further boost the utility of Ad vectors by enhancing the safety, efficacy and duration of transgene expression.
Figures

References
-
- Akbulut H, Zhang L, Tang Y, Deisseroth A. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther. 2003;10:388–395. - PubMed
-
- Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. - PubMed
-
- Ambar BB, Frei K, Malipiero U, Morelli AE, Castro MG, Lowenstein PR, Fontana A. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648. - PubMed
-
- Aoki Y, Hosaka S, Kawa S, Kiyosawa K. Potential tumor-targeting peptide vector of histidylated oligolysine conjugated to a tumor-homing RGD motif. Cancer Gene Ther. 2001;8:783–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

