Recent advances in azo dye degrading enzyme research
- PMID: 16611136
- PMCID: PMC5863238
- DOI: 10.2174/138920306776359786
Recent advances in azo dye degrading enzyme research
Abstract
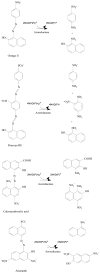
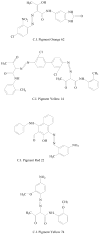
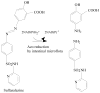
Azo dyes, which are characterized by one or more azo bonds, are a predominant class of colorants used in tattooing, cosmetics, foods, and consumer products. These dyes are mainly metabolized by bacteria to colorless aromatic amines, some of which are carcinogenic, by azoreductases that catalyze a NAD(P)H-dependent reduction. The resulting amines are further degraded aerobically by bacteria. Some bacteria have the ability to degrade azo dyes both aerobically and anaerobically. Plant-degrading white rot fungi can break down azo dyes by utilizing a number of oxidases and peroxidases as well. In yeast, a ferric reductase system participates in the extracellular reduction of azo dyes. Recently, two types of azoreductases have been discovered in bacteria. The first class of azoreductases is monomeric flavin-free enzymes containing a putative NAD(P)H binding motif at their N-termini; the second class is polymeric flavin dependent enzymes which are studied more extensively. Azoreductases from bacteria represent novel families of enzymes with little similarity to other reductases. Dissociation and reconstitution of the flavin dependent azoreductases demonstrate that the non-covalent bound flavin prosthetic group is required for the enzymatic functions. In this review, structures and carcinogenicity of azo colorants, protein structure, enzymatic function, and substrate specificity, as well as application of the azo dyes and azoreductases will be discussed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
