Degenerative suspensory ligament desmitis as a systemic disorder characterized by proteoglycan accumulation
- PMID: 16611357
- PMCID: PMC1459153
- DOI: 10.1186/1746-6148-2-12
Degenerative suspensory ligament desmitis as a systemic disorder characterized by proteoglycan accumulation
Abstract
Background: Degenerative suspensory ligament desmitis (DSLD) is a debilitating disorder thought to be limited to suspensory ligaments of Peruvian Pasos, Peruvian Paso crosses, Arabians, American Saddlebreds, American Quarter Horses, Thoroughbreds, and some European breeds. It frequently leads to persistent, incurable lameness and need to euthanize affected horses. The pathogenesis remains unclear, though the disease appears to run in families. Treatment and prevention are empirical and supportive, and not effective in halting the progression of the disease. Presently, the presumptive diagnosis of DSLD is obtained from patient signalment and history, clinical examination, and ultrasonographic examination of clinically affected horses, and is confirmed at post mortem examination. Presently, there are no reliable methods of diagnosing DSLD in asymptomatic horses. The goal of this study was to characterize and define the disorder in terms of tissue involvement at the macroscopic and microscopic levels.
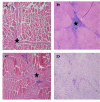
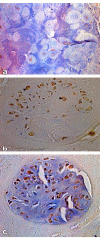
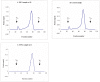
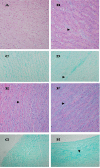
Results: We examined tissues and organs from 28 affected horses (22 Peruvian Pasos, 6 horses of other breeds) and from 8 control horses. Histopathological examination revealed the presence of excessive amounts of proteoglycans in the following tissues removed from DSLD-affected horses: suspensory ligaments, superficial and deep digital flexor tendons, patellar and nuchal ligaments, cardiovascular system, and sclerae. Electron microscopy demonstrated changes in diameters of collagen fibrils in the tendon, and in smooth muscle cells of the media of the aorta compatible with increased cell permeability in DSLD-affected cells. Separation of tendon extracts by gel chromatography revealed the presence of additional proteoglycan(s) in extracts from affected, but not control extracts.
Conclusion: This study demonstrates for the first time that DSLD, a disease process previously thought to be limited to the suspensory ligaments of the distal limbs of affected horses, is in fact a systemic disorder involving tissues and organs with significant connective tissue component. Abnormal accumulation of proteoglycans between collagen and elastic fibers rather than specific collagen fibril abnormalities is the most prominent histological feature of DSLD. Because of this observation and because of the involvement of many other tendons and ligaments beside the suspensory ligament, and of non-ligamentous tissue we, therefore, propose that equine systemic proteoglycan accumulation or ESPA rather than DSLD is a more appropriate name for this condition.
Figures


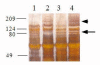

References
-
- Dowling BA, Dart AJ, Hodgson DR, Smith RKW. Superficial digital flexor tendonitis in the horse. Equine Vet J. 2000;32:369–378. - PubMed
-
- Genovese RL. Prognosis of superficial digital flexor tendon and suspensory ligament injuries. Proc Am Assoc Equine Pract. 1993. pp. 17–19.
-
- Goodship AE. The pathophysiology of flexor tendon injury in the horse. Equine Vet Educ. 1993;5:23–29.
-
- Peloso JG, Mundy GD, Cohen ND. Prevalence of, and factors associated with musculoskeletal racing injuries of Thoroughbreds. J Am Vet Med Assoc. 1994;204:620–626. - PubMed
-
- Bramlage LR, Hogan PM. Career results of 137 Thoroughbred racehorses that have undergone superior check ligament desmotomy for treatment of tendinitis. Proc Am Assoc Equine Pract. 1996. pp. 162–163.
LinkOut - more resources
Full Text Sources

