Prostate tumor growth and recurrence can be modulated by the omega-6:omega-3 ratio in diet: athymic mouse xenograft model simulating radical prostatectomy
- PMID: 16611404
- PMCID: PMC1578514
- DOI: 10.1593/neo.05637
Prostate tumor growth and recurrence can be modulated by the omega-6:omega-3 ratio in diet: athymic mouse xenograft model simulating radical prostatectomy
Abstract
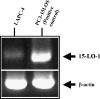
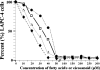
Evidence indicates that a diet rich in omega (omega)-6 polyunsaturated fatty acids (PUFAs) [e.g., linoleic acid (LA)] increases prostate cancer (PCa) risk, whereas a diet rich in omega-3 decreases risk. Precisely how these PUFAs affect disease development remains unclear. So we examined the roles that PUFAs play in PCa, and we determined if increased omega-3 consumption can impede tumor growth. We previously demonstrated an increased expression of an omega-6 LA-metabolizing enzyme, 15-lipoxygenase-1 (15-LO-1, ALOX15), in prostate tumor tissue compared with normal adjacent prostate tissue, and that elevated 15-LO-1 activity in PCa cells has a protumorigenic effect. A PCa cell line, Los Angeles Prostate Cancer-4 (LAPC-4), expresses prostate-specific antigen (PSA) as well an active 15-LO-1 enzyme. Therefore, to study whether or not the protumorigenic role of 15-LO-1 and dietary omega-6 LA can be modulated by altering omega-3 levels through diet, we surgically removed tumors caused by LAPC-4 cells (mouse model to simulate radical prostatectomy). Mice were then randomly divided into three different diet groups-namely, high omega-6 LA, high omega-3 stearidonic acid (SDA), and no fat-and examined the effects of omega-6 and omega-3 fatty acids in diet on LAPC-4 tumor recurrence by monitoring for PSA. Mice in these diet groups were monitored for food consumption, body weight, and serum PSA indicative of the presence of LAPC-4 cells. Fatty acid methyl esters from erythrocyte membranes were examined for omega-6 and omega-3 levels to reflect long-term dietary intake. Our results provide evidence that prostate tumors can be modulated by the manipulation of omega-6:omega-3 ratios through diet and that the omega-3 fatty acid SDA [precursor of eicosapentaenoic acid (EPA)] promotes apoptosis and decreases proliferation in cancer cells, causing decreased PSA doubling time, compared to omega-6 LA fatty acid, likely by competing with the enzymes of LA and AA pathways, namely, 15-LO-1 and cyclooxygenases (COXs). Thus, EPA and DHA (major components of fish oil) could potentially be promising dietary intervention agents in PCa prevention aimed at 15-LO-1 and COX-2 as molecular targets. These observations also provide clues as to its mechanisms of action.
Figures


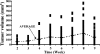





References
-
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. - PubMed
-
- Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH, et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20:680–688. - PubMed
-
- Dunn JE. Cancer epidemiology in populations of the United States—with emphasis on Hawaii and California—and Japan. Cancer Res. 1975;35:3240–3245. - PubMed
-
- Haenszel W, Kurihara M. Studies of Japanese migrants: I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. - PubMed
-
- Shennen DH, Bishop OS. Diet and mortality from malignant disease in 32 countries. West Indian Med J. 1974;23:44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
