Imaging-guided acute ischemic stroke therapy: From "time is brain" to "physiology is brain"
- PMID: 16611754
- PMCID: PMC8133997
Imaging-guided acute ischemic stroke therapy: From "time is brain" to "physiology is brain"
Abstract
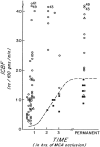
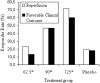
The number of potential patients who are actually treated for acute ischemic stroke is disappointingly low, and effective treatments are making a minor impact on this major public health problem. Imaging is not regularly used to identify the ischemic penumbra, a key concept in stroke physiology, though it is capable of doing so in a clinically relevant manner. Evidence is accumulating that identification of the ischemic penumbra and making treatment decisions on the basis of its presence provide substantial benefit to patient outcomes. Moreover, the same studies suggest that an unexpectedly large proportion of patients are suitable for therapy well past the traditional time windows because of the existence of a substantial ischemic penumbra. Modern MR imaging and CT systems, now widely available, are capable of answering the most relevant physiologic questions in acute ischemic stroke. This capability presents new opportunities and responsibilities to neuroradiologists to make appropriate imaging readily available and to have the imaging data rapidly processed and interpreted. In this article, acute ischemic stroke therapy, including the role of imaging in current medical practice, is reviewed, and an evidence-based alternative to contemporary acute ischemic stroke therapy is suggested.
Figures




References
-
- NINDS Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–87 - PubMed
-
- Gonzalez RG, Hirsch J, Koroshetz W, et al. Acute Ischemic Stroke: Imaging and Intervention. Berlin, Germany: Springer-Verlag; 2006
-
- Lisboa RC, Jovanovic BD, Alberts MJ. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke 2002;33:2866–71 - PubMed
-
- Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 - PubMed
-
- Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
