Base pairing between the 5' half of epsilon and a cis-acting sequence, phi, makes a contribution to the synthesis of minus-strand DNA for human hepatitis B virus
- PMID: 16611897
- PMCID: PMC1471998
- DOI: 10.1128/JVI.80.9.4380-4387.2006
Base pairing between the 5' half of epsilon and a cis-acting sequence, phi, makes a contribution to the synthesis of minus-strand DNA for human hepatitis B virus
Abstract
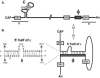
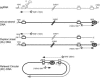
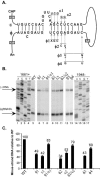
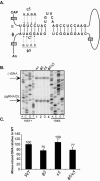
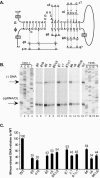
Synthesis of minus-strand DNA of human hepatitis B virus (HBV) can be divided into three phases: initiation of DNA synthesis, the template switch, and elongation of minus-strand DNA. Although much is known about minus-strand DNA synthesis, the mechanism(s) by which this occurs has not been completely elucidated. Through a deletion analysis, we have identified a cis-acting element involved in minus-strand DNA synthesis that lies within a 27-nucleotide region between DR2 and the 3' copy of DR1. A subset of this region (termed Phi) has been hypothesized to base pair with the 5' half of epsilon (H. Tang and A. McLachlan, Virology, 303:199-210, 2002). To test the proposed model, we used a genetic approach in which multiple sets of variants that disrupted and then restored putative base pairing between the 5' half of epsilon and phi were analyzed. Primer extension analysis, using two primers simultaneously, was performed to measure encapsidated pregenomic RNA (pgRNA) and minus-strand DNA synthesized in cell culture. The efficiency of minus-strand DNA synthesis was defined as the amount of minus-strand DNA synthesized per encapsidation event. Our results indicate that base pairing between phi and the 5' half of epsilon contributes to efficient minus-strand DNA synthesis. Additional results are consistent with the idea that the primary sequence of phi and/or epsilon also contributes to function. How base pairing between phi and epsilon contributes to minus-strand DNA synthesis is not known, but a simple speculation is that phi base pairs with the 5' half of epsilon to juxtapose the donor and acceptor sites to facilitate the first-strand template switch.
Figures

References
-
- Beasley, R. P. 1988. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer 61:1942-1956. - PubMed
-
- Ganem, D., and R. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-3036. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

