In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1
- PMID: 16611904
- PMCID: PMC1472006
- DOI: 10.1128/JVI.80.9.4440-4446.2006
In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1
Abstract
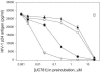
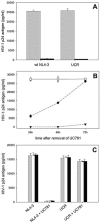
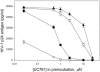
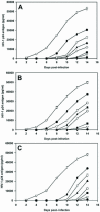
The nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 is under development as a microbicide to prevent sexual transmission of the human immunodeficiency virus type 1 (HIV-1). However, NNRTI-resistant HIV-1 is increasingly prevalent in the infected population, and one of the concerns for NNRTI-based microbicides is that they will be ineffective against drug-resistant virus and may in fact selectively transmit NNRTI-resistant virus. We evaluated the microbicidal activity of UC781 against UC781-resistant (UCR), efavirenz-resistant (EFVR), and nevirapine-resistant (NVPR) strains in a variety of microbicide-relevant tests, including inactivation of cell-free virus, inhibition of cell-to-cell HIV-1 transmission, and the ability of UC781 pretreatment to protect cells from subsequent infection in the absence of exogenous drug. UC781 was 10- to 100-fold less effective against NNRTI-resistant HIV-1 compared to wild-type (wt) virus in each of these tests, with UC781 microbicidal activity against the various virus strains being wt > or = NVPR > UCR > or = EFVR. Breakthrough experiments using UC781-pretreated cells and mixtures of wt and NNRTI-resistant HIV-1 showed that UC781-pretreatment selected for NNRTI-resistant HIV-1. However, the efficacy of UC781 was dose dependent, and 25 microM UC781 provided essentially equivalent microbicidal activity against NNRTI-resistant and wt virus. The amount of UC781 in topical microbicide formulations under current development is approximately 100-fold greater than this concentration, so transmission of NNRTI-resistant virus may not be an issue at these microbicide formulation levels of UC781. Nonetheless, the reduced microbicidal activity of UC781 against NNRTI-resistant HIV-1 suggests that additional antiviral agents should be included in NNRTI-based microbicide formulations.
Figures
Similar articles
-
Human immunodeficiency virus type 1 resistance or cross-resistance to nonnucleoside reverse transcriptase inhibitors currently under development as microbicides.Antimicrob Agents Chemother. 2011 Apr;55(4):1403-13. doi: 10.1128/AAC.01426-10. Epub 2011 Jan 31. Antimicrob Agents Chemother. 2011. PMID: 21282453 Free PMC article.
-
Attenuated infectivity of HIV type 1 from epithelial cells pretreated with a tight-binding nonnucleoside reverse transcriptase inhibitor.AIDS Res Hum Retroviruses. 2002 Jul 1;18(10):711-4. doi: 10.1089/088922202760072339. AIDS Res Hum Retroviruses. 2002. PMID: 12167278
-
Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase.J Virol. 1997 Apr;71(4):3023-30. doi: 10.1128/JVI.71.4.3023-3030.1997. J Virol. 1997. PMID: 9060662 Free PMC article.
-
The emerging profile of cross-resistance among the nonnucleoside HIV-1 reverse transcriptase inhibitors.Viruses. 2014 Jul 31;6(8):2960-73. doi: 10.3390/v6082960. Viruses. 2014. PMID: 25089538 Free PMC article. Review.
-
HIV-1 RT nonnucleoside inhibitors and their interaction with RT for antiviral drug development.Infect Disord Drug Targets. 2006 Dec;6(4):391-413. doi: 10.2174/187152606779025833. Infect Disord Drug Targets. 2006. PMID: 17168804 Review.
Cited by
-
Structural and inhibition studies of the RNase H function of xenotropic murine leukemia virus-related virus reverse transcriptase.Antimicrob Agents Chemother. 2012 Apr;56(4):2048-61. doi: 10.1128/AAC.06000-11. Epub 2012 Jan 17. Antimicrob Agents Chemother. 2012. PMID: 22252812 Free PMC article.
-
Development and Characterization of a Vaginal Film Containing Dapivirine, a Non- nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission.Drug Deliv Transl Res. 2011 Jun 1;1(3):209-222. doi: 10.1007/s13346-011-0022-6. Drug Deliv Transl Res. 2011. PMID: 22708075 Free PMC article.
-
Characterization of UC781-tenofovir combination gel products for HIV-1 infection prevention in an ex vivo ectocervical model.Antimicrob Agents Chemother. 2012 Jun;56(6):3058-66. doi: 10.1128/AAC.06284-11. Epub 2012 Mar 19. Antimicrob Agents Chemother. 2012. PMID: 22430977 Free PMC article.
-
Human immunodeficiency virus type 1 resistance or cross-resistance to nonnucleoside reverse transcriptase inhibitors currently under development as microbicides.Antimicrob Agents Chemother. 2011 Apr;55(4):1403-13. doi: 10.1128/AAC.01426-10. Epub 2011 Jan 31. Antimicrob Agents Chemother. 2011. PMID: 21282453 Free PMC article.
-
Targeting Trojan Horse leukocytes for HIV prevention.AIDS. 2010 Jan 16;24(2):163-87. doi: 10.1097/QAD.0b013e32833424c8. AIDS. 2010. PMID: 20010071 Free PMC article. Review. No abstract available.
References
-
- Abner, S. R., P. C. Guenthner, J. Guarner, K. A. Hancock, J. E. Cummins, A. Fink, G. T. Gilmore, C. Staley, A. Ward, O. Ali, S. Binderow, S. Cohen, L. A. Grohskopf, L. Paxton, C. E. Hart, and C. S. Dezzutti. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 192:1545-1556. - PubMed
-
- Almond, L. M., P. G. Hoggard, D. Edirisinghe, S. H. Khoo, and D. J. Back. 2005. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. J. Antimicrob. Chemother. 56:738-744. - PubMed
-
- Ambrose, Z., V. Boltz, S. Palmer, J. M. Coffin, S. H. Hughes, and V. N. KewalRamani. 2004. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J. Virol. 78:13553-13561. - PMC - PubMed
-
- Andreoletti, L., N. Chomont, G. Gresenguet, M. Matta, J. de Dieu Longo, M. P. Carreno, A. Si-Mohamed, J. Legoff, M. D. Kazatchkine, and L. Belec. 2003. Independent levels of cell-free and cell-associated human immunodeficiency virus-1 in genital-tract secretions of clinically asymptomatic, treatment-naive African women. J. Infect. Dis. 188:549-554. - PubMed
-
- Bacheler, L. T., E. D. Anton, P. Kudish, D. Baker, J. Bunville, K. Krakowski, L. Bolling, M. Aujay, X. V. Wang, D. Ellis, M. F. Becker, A. L. Lasut, H. J. George, D. R. Spalding, G. Hollis, and K. Abremski. 2000. Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob. Agents Chemother. 44:2475-2484. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

