Prion infection of oral and nasal mucosa
- PMID: 16611915
- PMCID: PMC1472028
- DOI: 10.1128/JVI.80.9.4546-4556.2006
Prion infection of oral and nasal mucosa
Abstract
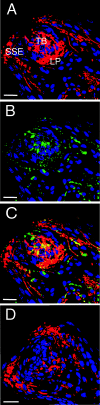
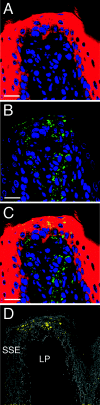
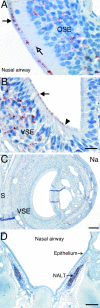
Centrifugal spread of the prion agent to peripheral tissues is postulated to occur by axonal transport along nerve fibers. This study investigated the distribution of the pathological isoform of the protein (PrP(Sc)) in the tongues and nasal cavities of hamsters following intracerebral inoculation of the HY strain of the transmissible mink encephalopathy (TME) agent. We report that PrP(Sc) deposition was found in the lamina propria, taste buds, and stratified squamous epithelium of fungiform papillae in the tongue, as well as in skeletal muscle cells. Using laser scanning confocal microscopy, PrP(Sc) was localized to nerve fibers in each of these structures in the tongue, neuroepithelial taste cells of the taste bud, and, possibly, epithelial cells. This PrP(Sc) distribution was consistent with a spread of HY TME agent along both somatosensory and gustatory cranial nerves to the tongue and suggests subsequent synaptic spread to taste cells and epithelial cells via peripheral synapses. In the nasal cavity, PrP(Sc) accumulation was found in the olfactory and vomeronasal epithelium, where its location was consistent with a distribution in cell bodies and apical dendrites of the sensory neurons. Prion spread to these sites is consistent with transport via the olfactory nerve fibers that descend from the olfactory bulb. Our data suggest that epithelial cells, neuroepithelial taste cells, or olfactory sensory neurons at chemosensory mucosal surfaces, which undergo normal turnover, infected with the prion agent could be shed and play a role in the horizontal transmission of animal prion diseases.
Figures

Similar articles
-
Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa.J Virol. 2009 Jul;83(13):6435-45. doi: 10.1128/JVI.00018-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369351 Free PMC article.
-
Prion shedding from olfactory neurons into nasal secretions.PLoS Pathog. 2010 Apr 15;6(4):e1000837. doi: 10.1371/journal.ppat.1000837. PLoS Pathog. 2010. PMID: 20419120 Free PMC article.
-
Prion infection of skeletal muscle cells and papillae in the tongue.J Virol. 2004 Jul;78(13):6792-8. doi: 10.1128/JVI.78.13.6792-6798.2004. J Virol. 2004. PMID: 15194754 Free PMC article.
-
The Role of the Nasal Cavity in the Pathogenesis of Prion Diseases.Viruses. 2021 Nov 16;13(11):2287. doi: 10.3390/v13112287. Viruses. 2021. PMID: 34835094 Free PMC article. Review.
-
Transmission and Replication of Prions.Prog Mol Biol Transl Sci. 2017;150:181-201. doi: 10.1016/bs.pmbts.2017.06.014. Epub 2017 Aug 7. Prog Mol Biol Transl Sci. 2017. PMID: 28838661 Review.
Cited by
-
Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa.J Virol. 2009 Jul;83(13):6435-45. doi: 10.1128/JVI.00018-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369351 Free PMC article.
-
Prion shedding from olfactory neurons into nasal secretions.PLoS Pathog. 2010 Apr 15;6(4):e1000837. doi: 10.1371/journal.ppat.1000837. PLoS Pathog. 2010. PMID: 20419120 Free PMC article.
-
Aerosol and nasal transmission of chronic wasting disease in cervidized mice.J Gen Virol. 2010 Jun;91(Pt 6):1651-8. doi: 10.1099/vir.0.017335-0. Epub 2010 Feb 17. J Gen Virol. 2010. PMID: 20164261 Free PMC article.
-
Pathogenesis of chronic wasting disease in cervidized transgenic mice.Am J Pathol. 2010 Jun;176(6):2785-97. doi: 10.2353/ajpath.2010.090710. Epub 2010 Apr 15. Am J Pathol. 2010. PMID: 20395435 Free PMC article.
-
Intra-host mathematical model of chronic wasting disease dynamics in deer (Odocoileus).Prion. 2016 Sep 2;10(5):377-390. doi: 10.1080/19336896.2016.1189054. Epub 2016 Aug 18. Prion. 2016. PMID: 27537196 Free PMC article.
References
-
- Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J. M. Elsen, and F. Lantier. 2000. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. - PubMed
-
- Andreoletti, O., C. Lacroux, A. Chabert, L. Monnereau, G. Tabouret, F. Lantier, P. Berthon, F. Eychenne, S. Lafond-Benestad, J. M. Elsen, and F. Schelcher. 2002. PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83:2607-2616. - PubMed
-
- Andreoletti, O., S. Simon, C. Lacroux, N. Morel, G. Tabouret, A. Chabert, S. Lugan, F. Corbiere, P. Ferre, G. Foucras, H. Laude, F. Eychenne, J. Grassi, and F. Schelcher. 2004. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat. Med. 10:591-593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

