Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response
- PMID: 16611919
- PMCID: PMC1471997
- DOI: 10.1128/JVI.80.9.4591-4600.2006
Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response
Abstract
Human cytomegalovirus (HCMV) is a ubiquitous human pathogen that infects 40 to 90% of adult human populations. HCMV infections are often asymptomatic in healthy individuals but can cause severe organ and life-threatening disease in immunocompromised patients. The antiviral antibody response to HCMV infection is complex and is known to include virus-neutralizing antibody production against surface glycoproteins encoded by HCMV. We have investigated the human antibody response to a complex of HCMV surface glycoproteins composed of glycoprotein M (gM)/gN, the gene products of the UL100 and UL73 open reading frames. Mouse monoclonal antibodies generated against gM/gN have previously been shown to neutralize HCMV infection of human fibroblasts in vitro. To determine whether human antibodies reactive with the gM/gN complex possess virus-neutralizing properties, we isolated human antibodies reactive with gM/gN from pooled human HCMV hyperimmune globulin by affinity purification using recombinant gM/gN. The affinity-purified human anti-gM/gN antibodies reacted specifically by immunofluorescence with HCMV-infected human fibroblasts and with cells transiently expressing gM/gN, but not with cells transfected with plasmids encoding other immunogenic HCMV proteins. The anti-gM/gN antibodies also reacted specifically only with gM/gN in immunoblot assays using lysates of transfected cells expressing specific HCMV proteins. Last, human anti-gM/gN antibodies efficiently neutralized infectious HCMV in vitro with a capacity comparable to that of human anti-gB antibodies. These data indicated that gM/gN can elicit a virus-neutralizing antibody response in humans infected with HCMV and therefore should be considered a potential candidate for inclusion in prophylactic CMV vaccines.
Figures
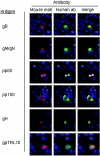
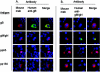


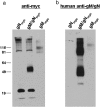
References
-
- Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. - PubMed
-
- Arvin, A. M., P. Fast, M. Myers, S. Plotkin, and R. Rabinovich. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233-239. - PubMed
-
- Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. - PubMed
-
- Britt, W. J., and D. Auger. 1985. Identification of a 65,000 dalton virion envelope of human cytomegalovirus. Virus Res. 4:31-36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

