Chromosomal distribution of PcG proteins during Drosophila development
- PMID: 16613483
- PMCID: PMC1440717
- DOI: 10.1371/journal.pbio.0040170
Chromosomal distribution of PcG proteins during Drosophila development
Abstract
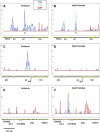
Polycomb group (PcG) proteins are able to maintain the memory of silent transcriptional states of homeotic genes throughout development. In Drosophila, they form multimeric complexes that bind to specific DNA regulatory elements named PcG response elements (PREs). To date, few PREs have been identified and the chromosomal distribution of PcG proteins during development is unknown. We used chromatin immunoprecipitation (ChIP) with genomic tiling path microarrays to analyze the binding profile of the PcG proteins Polycomb (PC) and Polyhomeotic (PH) across 10 Mb of euchromatin. We also analyzed the distribution of GAGA factor (GAF), a sequence-specific DNA binding protein that is found at most previously identified PREs. Our data show that PC and PH often bind to clustered regions within large loci that encode transcription factors which play multiple roles in developmental patterning and in the regulation of cell proliferation. GAF co-localizes with PC and PH to a limited extent, suggesting that GAF is not a necessary component of chromatin at PREs. Finally, the chromosome-association profile of PC and PH changes during development, suggesting that the function of these proteins in the regulation of some of their target genes might be more dynamic than previously anticipated.
Figures
Comment in
-
Polycombing Drosophila for dynamic developmental footprints.PLoS Biol. 2006 Jun;4(6):e177. doi: 10.1371/journal.pbio.0040177. Epub 2006 Apr 20. PLoS Biol. 2006. PMID: 20076582 Free PMC article. No abstract available.
References
-
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. - PubMed
-
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. - PubMed
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

