Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat
- PMID: 16613874
- PMCID: PMC1779756
- DOI: 10.1113/jphysiol.2006.108514
Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat
Abstract
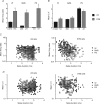
Through their widespread projections to the entire brain, dorsal raphe cells participate in many physiological functions and are associated with neuropsychiatric disorders. In previous studies, the width of action potentials was used as a criterion to identify putative serotonergic neurons, and to demonstrate that cells with broad spikes were more active in wakefulness, slowed down their activity in slow wave sleep and became virtually silent during paradoxical sleep. However, recent studies reported that about half of these presumed serotonergic cells were not immunoreactive for tyrosine hydroxylase. Here, we re-examine the electrophysiological properties of dorsal raphe cells across the sleep-wake cycle in rats by the extracellular recording of a large sample of single units (n = 770). We identified two major types of cells, which differ in spike waveform: a first population characterized by broad, mostly positive spikes, and a second one displaying symmetrical positive-negative spikes with a large distribution of spike durations (0.6-3.2 ms). Although we found classical broad-spike cells that were more active in wakefulness, we also found that about one-third of these cells increased or did not change their firing rate during sleep compared with wakefulness. Moreover, 62% of the latter cells were active in paradoxical sleep when most of raphe cells were silent. Such a diversity in the neuronal firing behaviour is important in the light of the recent controversy regarding the neurochemical identity of dorsal raphe cells exhibiting broad spikes. Our results also suggest that the dorsal raphe contains subpopulations of neurons with reciprocal activity across the sleep-wake cycle.
Figures






References
-
- Adrien J, Lanfumey L. Ontogenesis of unit activity in the raphe dorsalis of the behaving kitten: its relationship with the states of vigilance. Brain Res. 1986;366:10–21. - PubMed
-
- Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. - PubMed
-
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. - PubMed
-
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
-
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

