Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice
- PMID: 16613881
- PMCID: PMC1779746
- DOI: 10.1113/jphysiol.2006.106914
Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice
Abstract
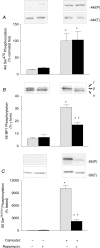
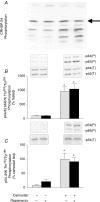
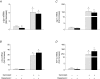
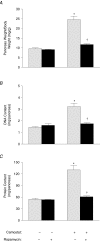
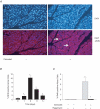
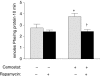
Cholecystokinin (CCK)-induced pancreatic growth in mice involves parallel increases in DNA and protein. The mammalian target of rapamycin (mTOR) signalling pathway regulates mRNA translation and its activation is implicated in growth of various tissues. The aim of this study was to elucidate whether mTOR activation is required for pancreatic growth in a mouse model of increased endogenous CCK release. In mice fed chow containing the synthetic protease inhibitor camostat, protein synthetic rates and phosphorylation of two downstream targets of mTOR, eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and the ribosomal protein S6 (S6), increased in comparison with fasted controls. The camostat-induced increases in protein synthesis and 4E-BP1 and S6 phosphorylation were almost totally abolished by administration of the mTOR inhibitor rapamycin 1 h prior to camostat feeding. In contrast, the phosphorylation of ERK1/2 and JNK and the expression of the early response genes c-jun, c-fos, ATF3 and egr-1 induced by camostat feeding were not affected by rapamycin. In mice fed camostat for 7 days, the ratio of pancreatic to body weight increased by 143%, but when rapamycin was administered daily this was reduced to a 22% increase. Changes in pancreatic mass were paralleled by protein and DNA content following camostat feeding and rapamycin administration. Moreover, while BrdU incorporation, an indicator of DNA synthesis, was increased to 448% of control values after 2 days of camostat feeding, rapamycin administration completely inhibited this increase. We conclude that the mTOR signalling pathway is required for CCK-induced cell division and pancreatic growth.
Figures
Similar articles
-
CCK-induced pancreatic growth is not limited by mitogenic capacity in mice.Am J Physiol Gastrointest Liver Physiol. 2008 May;294(5):G1148-57. doi: 10.1152/ajpgi.00426.2007. Epub 2008 Mar 20. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18356533
-
Calcineurin mediates pancreatic growth in protease inhibitor-treated mice.Am J Physiol Gastrointest Liver Physiol. 2004 May;286(5):G784-90. doi: 10.1152/ajpgi.00446.2003. Epub 2003 Dec 18. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 14684381
-
Induction of early response genes in trypsin inhibitor-induced pancreatic growth.Am J Physiol Gastrointest Liver Physiol. 2007 Feb;292(2):G667-77. doi: 10.1152/ajpgi.00433.2006. Epub 2006 Nov 9. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17095753
-
The molecular target of rapamycin (mTOR) as a therapeutic target against cancer.Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S169-77. Cancer Biol Ther. 2003. PMID: 14508096 Review.
-
Altered protein synthesis is a trigger for long-term memory formation.Neurobiol Learn Mem. 2008 Mar;89(3):247-59. doi: 10.1016/j.nlm.2007.08.009. Epub 2007 Oct 4. Neurobiol Learn Mem. 2008. PMID: 17919940 Free PMC article. Review.
Cited by
-
Loss of LAT1 sex-dependently delays recovery after caerulein-induced acute pancreatitis.World J Gastroenterol. 2022 Mar 14;28(10):1024-1054. doi: 10.3748/wjg.v28.i10.1024. World J Gastroenterol. 2022. PMID: 35431492 Free PMC article.
-
Profiling CCK-mediated pancreatic growth: the dynamic genetic program and the role of STATs as potential regulators.Physiol Genomics. 2012 Jan 18;44(1):14-24. doi: 10.1152/physiolgenomics.00255.2010. Epub 2011 Oct 18. Physiol Genomics. 2012. PMID: 22010007 Free PMC article.
-
Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis.Autophagy. 2019 Nov;15(11):1954-1969. doi: 10.1080/15548627.2019.1596486. Epub 2019 Mar 30. Autophagy. 2019. PMID: 30894069 Free PMC article.
-
CCK-independent mTORC1 activation during dietary protein-induced exocrine pancreas growth.Am J Physiol Gastrointest Liver Physiol. 2010 Nov;299(5):G1154-63. doi: 10.1152/ajpgi.00445.2009. Epub 2010 Aug 26. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20798356 Free PMC article.
-
Secretin is not necessary for exocrine pancreatic development and growth in mice.Am J Physiol Gastrointest Liver Physiol. 2011 Nov;301(5):G791-8. doi: 10.1152/ajpgi.00245.2011. Epub 2011 Aug 18. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21852360 Free PMC article.
References
-
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. - PubMed
-
- Bi Y, Williams JA. Receptor biology and signal transduction in pancreatic acinar cells. Curr Opin Gastroenterol. 2004;20:427–434. - PubMed
-
- Bragado MJ, Groblewski GE, Williams JA. p70s6k is activated by CCK in rat pancreatic acini. Am J Physiol. 1997;273:C101–C109. - PubMed
-
- Bragado MJ, Groblewski GE, Williams JA. Regulation of protein synthesis by cholecystokinin in rat pancreatic acini involves PHAS-I and the p70, S6 kinase pathway. Gastroenterology. 1998;115:733–742. - PubMed
-
- Chernick SS, Lepkovsky S, Chaikoff IL. A dietary factor regulating the enzyme content of the pancreas: Changes induced in size and proteolytic activity of the chick pancreas by the ingestion of raw soy-bean meal. Am J Physiol. 1948;155:33–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

