Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155
- PMID: 16614444
- PMCID: PMC1435982
- DOI: 10.1093/nar/gkl143
Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155
Abstract
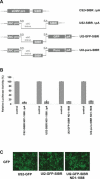
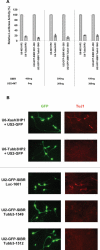
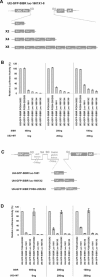
Vector-based RNA interference (RNAi) has emerged as a valuable tool for analysis of gene function. We have developed new RNA polymerase II expression vectors for RNAi, designated SIBR vectors, based upon the non-coding RNA BIC. BIC contains the miR-155 microRNA (miRNA) precursor, and we find that expression of a short region of the third exon of mouse BIC is sufficient to produce miR-155 in mammalian cells. The SIBR vectors use a modified miR-155 precursor stem-loop and flanking BIC sequences to express synthetic miRNAs complementary to target RNAs. Like RNA polymerase III driven short hairpin RNA vectors, the SIBR vectors efficiently reduce target mRNA and protein expression. The synthetic miRNAs can be expressed from an intron, allowing coexpression of a marker or other protein with the miRNAs. In addition, intronic expression of a synthetic miRNA from a two intron vector enhances RNAi. A SIBR vector can express two different miRNAs from a single transcript for effective inhibition of two different target mRNAs. Furthermore, at least eight tandem copies of a synthetic miRNA can be expressed in a polycistronic transcript to increase the inhibition of a target RNA. The SIBR vectors are flexible tools for a variety of RNAi applications.
Figures






References
-
- Filipowicz W. RNAi: the nuts and bolts of the RISC machine. Cell. 2005;122:17–20. - PubMed
-
- Sandy P., Ventura A., Jacks T. Mammalian RNAi: a practical guide. Biotechniques. 2005;39:215–224. - PubMed
-
- Murchison E.P., Hannon G.J. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr. Opin. Cell Biol. 2004;16:223–229. - PubMed
-
- Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. - PubMed
-
- Lau N.C., Lim le E.P., Weinstein E.G., Bartel da V.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

