The effective temperature of Peptide ions dissociated by sustained off-resonance irradiation collisional activation in fourier transform mass spectrometry
- PMID: 16614752
- PMCID: PMC1435862
- DOI: 10.1021/jp9833193
The effective temperature of Peptide ions dissociated by sustained off-resonance irradiation collisional activation in fourier transform mass spectrometry
Abstract
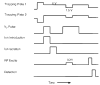
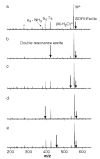
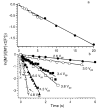
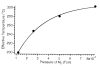
A method for determining the internal energy of biomolecule ions activated by collisions is demonstrated. The dissociation kinetics of protonated leucine enkephalin and doubly protonated bradykinin were measured using sustained off-resonance irradiation (SORI) collisionally activated dissociation (CAD) in a Fourier transform mass spectrometer. Dissociation rate constants are obtained from these kinetic data. In combination with Arrhenius parameters measured with blackbody infrared radiative dissociation, the "effective" temperatures of these ions are obtained. Effects of excitation voltage and frequency and the ion cell pressure were investigated. With typical SORI-CAD experimental conditions, the effective temperatures of these peptide ions range between 200 and 400 degrees C. Higher temperatures can be easily obtained for ions that require more internal energy to dissociate. The effective temperatures of both protonated leucine enkephalin and doubly protonated bradykinin measured with the same experimental conditions are similar. Effective temperatures for protonated leucine enkephalin can also be obtained from the branching ratio of the b(4) and (M + H - H(2)O)(+) pathways. Values obtained from this method are in good agreement with those obtained from the overall dissociation rate constants. Protonated leucine enkephalin is an excellent "thermometer" ion and should be well suited to establishing effective temperatures of ions activated by other dissociation techniques, such as infrared photodissociation, as well as ionization methods, such as matrix assisted laser desorption/ionization.
Figures








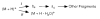
References
-
- McLafferty FW. Acc Chem Res. 1994;27:379–386.
-
- Valaskovic GA, Kelleher NL, McLafferty FW. Science. 1996;273:1199–1202. - PubMed
-
- Kelleher NL, Senko MW, Siegel MM, McLafferty FW. J Am Soc Mass Spectrom. 1997;8:380–383.
-
- Speir JP, Senko MW, Little DP, Loo JA, McLafferty FW. J Mass Spectrom. 1995;30:39–42.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
