Subpopulations of neurons expressing parvalbumin in the human amygdala
- PMID: 16615121
- PMCID: PMC1927834
- DOI: 10.1002/cne.20961
Subpopulations of neurons expressing parvalbumin in the human amygdala
Abstract
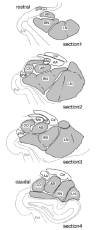
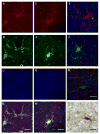
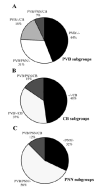
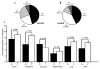
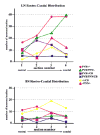
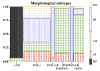
Amygdalar intrinsic inhibitory networks comprise several subpopulations of gamma-aminobutyric acidergic neurons, each characterized by distinct morphological features and clusters of functionally relevant neurochemical markers. In rodents, the calcium-binding proteins parvalbumin (PVB) and calbindin D28k (CB) are coexpressed in large subpopulations of amygdalar interneurons. PVB-immunoreactive (-IR) neurons have also been shown to be ensheathed by perineuronal nets (PNN), extracellular matrix envelopes believed to affect ionic homeostasis and synaptic plasticity. We tested the hypothesis that differential expression of these three markers may define distinct neuronal subpopulations within the human amygdala. Toward this end, triple-fluorescent labeling using antisera raised against PVB and CB as well as biotinylated Wisteria floribunda lectin for detection of PNN was combined with confocal microscopy. Among the 1,779 PVB-IR neurons counted, 18% also expressed CB, 31% were ensheathed in PNN, and 7% expressed both CB and PNN. Forty-four percent of PVB-IR neurons did not colocalize with either CB or PNN. The distribution of each of these neuronal subgroups showed substantial rostrocaudal gradients. Furthermore, distinct morphological features were found to characterize each neuronal subgroup. In particular, significant differences relative to the distribution and morphology were detected between PVB-IR neurons expressing CB and PVB-IR neurons wrapped in PNNs. These results indicate that amygdalar PVB-IR neurons can be subdivided into at least four different subgroups, each characterized by a specific neurochemical profile, morphological characteristics, and three-dimensional distribution. Such properties suggest that each of these neuronal subpopulations may play a specific role within the intrinsic circuitry of the amygdala.
Copyright 2006 Wiley-Liss, Inc.
Figures





Similar articles
-
Perineuronal nets labeled by monoclonal antibody VC1.1 ensheath interneurons expressing parvalbumin and calbindin in the rat amygdala.Brain Struct Funct. 2018 Apr;223(3):1133-1148. doi: 10.1007/s00429-017-1542-8. Epub 2017 Nov 1. Brain Struct Funct. 2018. PMID: 29094304 Free PMC article.
-
Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala.Neuroscience. 2001;105(3):681-93. doi: 10.1016/s0306-4522(01)00214-7. Neuroscience. 2001. PMID: 11516833
-
Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala.Brain Res. 2002 Jul 12;943(2):237-44. doi: 10.1016/s0006-8993(02)02650-1. Brain Res. 2002. PMID: 12101046
-
Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns.J Chem Neuroanat. 1999 Feb;16(2):77-116. doi: 10.1016/s0891-0618(98)00065-9. J Chem Neuroanat. 1999. PMID: 10223310 Review.
-
Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex.J Chem Neuroanat. 1997 Dec;14(1):1-19. doi: 10.1016/s0891-0618(97)10013-8. J Chem Neuroanat. 1997. PMID: 9498163 Review.
Cited by
-
The tetrapartite synapse: a key concept in the pathophysiology of schizophrenia.Eur Psychiatry. 2018 Apr;50:60-69. doi: 10.1016/j.eurpsy.2018.02.003. Epub 2018 Mar 2. Eur Psychiatry. 2018. PMID: 29503098 Free PMC article. Review.
-
Immunohistochemical characterization of parvalbumin-containing interneurons in the monkey basolateral amygdala.Neuroscience. 2009 Feb 18;158(4):1541-50. doi: 10.1016/j.neuroscience.2008.11.017. Epub 2008 Nov 17. Neuroscience. 2009. PMID: 19059310 Free PMC article.
-
Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala.Transl Psychiatry. 2015 Jan 20;5(1):e496. doi: 10.1038/tp.2014.128. Transl Psychiatry. 2015. PMID: 25603412 Free PMC article.
-
Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS.J Neurophysiol. 2011 Dec;106(6):2876-87. doi: 10.1152/jn.00312.2011. Epub 2011 Sep 7. J Neurophysiol. 2011. PMID: 21900512 Free PMC article.
-
Neuron-Glia Interactions in Neural Plasticity: Contributions of Neural Extracellular Matrix and Perineuronal Nets.Neural Plast. 2016;2016:5214961. doi: 10.1155/2016/5214961. Epub 2016 Jan 5. Neural Plast. 2016. PMID: 26881114 Free PMC article. Review.
References
-
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. - PubMed
-
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16:328–333. - PubMed
-
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992.
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

