Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine
- PMID: 16615128
- PMCID: PMC2823481
- DOI: 10.1002/cne.20962
Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine
Abstract
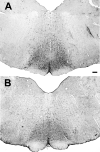
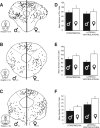
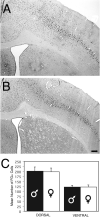
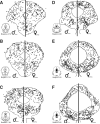
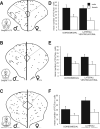
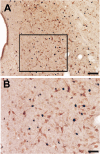
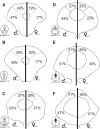
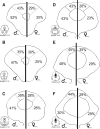
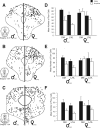
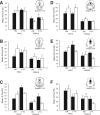
Previous studies have demonstrated that morphine, administered systemically or directly into the periaqueductal gray (PAG), produces a significantly greater degree of antinociception in males in comparison with females. Because the midbrain PAG and its descending projections to the rostral ventromedial medulla (RVM) constitute an essential neural circuit for opioid-based analgesia, the present studies were conducted to determine whether sex differences in the anatomical organization of the PAG-RVM pathway, and its activation during persistent inflammatory pain, could account for sex-based differences in opioid analgesia. In the rat, retrograde tracing was combined with Fos immunocytochemistry to investigate sexual dimorphism in the organization of the PAG-RVM circuit and its activation by persistent inflammatory pain induced by intraplantar injection of complete Freund's adjuvant (CFA). The ability of morphine to suppress the activation of the PAG-RVM circuit was also examined. Sexually dimorphic retrograde labeling was observed within the dorsomedial and lateral/ventrolateral PAG at all rostrocaudal levels, with females having significantly more PAG-RVM output neurons in comparison with males. While no sex differences were noted in the activation of the PAG by persistent inflammatory pain, significantly more PAG-RVM cells were activated in males in comparison with females. Systemic administration of morphine significantly suppressed CFA-induced Fos in the PAG in males only. The results of these studies demonstrate that both the anatomical organization and the functional activation of the PAG-RVM circuit are sexually dimorphic and may provide the anatomical substrate for sex-based differences in morphine analgesia.
Copyright 2006 Wiley-Liss, Inc.
Figures

Similar articles
-
Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception.Neuroscience. 2007 Jun 29;147(2):456-68. doi: 10.1016/j.neuroscience.2007.03.053. Epub 2007 May 31. Neuroscience. 2007. PMID: 17540508 Free PMC article.
-
Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat.Eur J Neurosci. 2008 Mar;27(6):1517-24. doi: 10.1111/j.1460-9568.2008.06100.x. Eur J Neurosci. 2008. PMID: 18364026 Free PMC article.
-
The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different?Neural Plast. 2009;2009:462879. doi: 10.1155/2009/462879. Epub 2009 Jan 28. Neural Plast. 2009. PMID: 19197373 Free PMC article. Review.
-
Sex differences in the amygdaloid projections to the ventrolateral periaqueductal gray and their activation during inflammatory pain in the rat.J Chem Neuroanat. 2022 Oct;124:102123. doi: 10.1016/j.jchemneu.2022.102123. Epub 2022 Jun 20. J Chem Neuroanat. 2022. PMID: 35738454
-
Neuronal and glial factors contributing to sex differences in opioid modulation of pain.Neuropsychopharmacology. 2019 Jan;44(1):155-165. doi: 10.1038/s41386-018-0127-4. Epub 2018 Jun 23. Neuropsychopharmacology. 2019. PMID: 29973654 Free PMC article. Review.
Cited by
-
Contextual control of conditioned pain tolerance and endogenous analgesic systems.Elife. 2022 Mar 11;11:e75283. doi: 10.7554/eLife.75283. Elife. 2022. PMID: 35275062 Free PMC article.
-
Examination of sex and minocycline treatment on acute morphine-induced analgesia and inflammatory gene expression along the pain pathway in Sprague-Dawley rats.Biol Sex Differ. 2015 Dec 12;6:33. doi: 10.1186/s13293-015-0049-3. eCollection 2015. Biol Sex Differ. 2015. PMID: 26693004 Free PMC article.
-
Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat.Physiol Behav. 2018 Apr 1;187:32-41. doi: 10.1016/j.physbeh.2017.11.030. Epub 2017 Dec 1. Physiol Behav. 2018. PMID: 29199028 Free PMC article.
-
Sex- and Gender-Based Pharmacological Response to Drugs.Pharmacol Rev. 2021 Apr;73(2):730-762. doi: 10.1124/pharmrev.120.000206. Pharmacol Rev. 2021. PMID: 33653873 Free PMC article.
-
PAG mu opioid receptor activation underlies sex differences in morphine antinociception.Behav Brain Res. 2007 Feb 12;177(1):126-33. doi: 10.1016/j.bbr.2006.10.028. Epub 2006 Nov 21. Behav Brain Res. 2007. PMID: 17118467 Free PMC article.
References
-
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. - PubMed
-
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. - PubMed
-
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. - PubMed
-
- Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. - PubMed
-
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

