Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide
- PMID: 16616566
- PMCID: PMC2587042
- DOI: 10.1016/j.ymgme.2006.02.012
Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide
Abstract
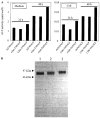
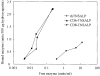
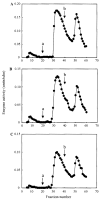
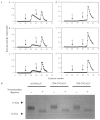
Hypophosphatasia is caused by deficiency of activity of the tissue-nonspecific alkaline phosphatase (TNSALP), resulting in a defect of bone mineralization. Enzyme replacement therapy (ERT) with partially purified plasma enzyme was attempted but with little clinical improvement. Attaining clinical effectiveness with ERT for hypophosphatasia may require delivering functional TNSALP enzyme to bone. We tagged the C-terminal-anchorless TNSALP enzyme with an acidic oligopeptide (a six or eight residue stretch of L-Asp), and compared the biochemical properties of the purified tagged and untagged enzymes derived from Chinese hamster ovary cell lines. The specific activities of the purified enzymes tagged with the acidic oligopeptide were the same as the untagged enzyme. In vitro affinity experiments showed the tagged enzymes had 30-fold higher affinity for hydroxyapatite than the untagged enzyme. Lectin affinity chromatography for carbohydrate structure showed little difference among the three enzymes. Biodistribution pattern from single infusion of the fluorescence-labeled enzymes into mice showed delayed clearance from the plasma up to 18 h post infusion and the amount of tagged enzyme retained in bone was 4-fold greater than that of the untagged enzyme. In vitro mineralization assays with the bone marrow from a hypophosphatasia patient using each of the three enzymes in the presence of high concentrations of pyrophosphate provided evidence of bone mineralization. These results show the anchorless enzymes tagged with an acidic oligopeptide are delivered efficiently to bone and function bioactively in bone mineralization, at least in vitro. They suggest potential advantages for use of these tagged enzymes in ERT for hypophosphatasia, which should be explored.
Figures



Similar articles
-
Enzyme replacement therapy on hypophosphatasia mouse model.J Inherit Metab Dis. 2014 Mar;37(2):309-317. doi: 10.1007/s10545-013-9646-7. Epub 2013 Aug 27. J Inherit Metab Dis. 2014. PMID: 23978959 Free PMC article.
-
Targeted drug delivery to bone: pharmacokinetic and pharmacological properties of acidic oligopeptide-tagged drugs.Curr Drug Discov Technol. 2008 Mar;5(1):39-48. doi: 10.2174/157016308783769405. Curr Drug Discov Technol. 2008. PMID: 18537566 Review.
-
Molecular basis of perinatal hypophosphatasia with tissue-nonspecific alkaline phosphatase bearing a conservative replacement of valine by alanine at position 406. Structural importance of the crown domain.FEBS J. 2008 Jun;275(11):2727-37. doi: 10.1111/j.1742-4658.2008.06414.x. Epub 2008 Apr 18. FEBS J. 2008. PMID: 18422967
-
[Improved Therapeutic Efficacy in Bone and Joint Disorders by Targeted Drug Delivery to Bone].Yakugaku Zasshi. 2016;136(11):1501-1508. doi: 10.1248/yakushi.16-00178. Yakugaku Zasshi. 2016. PMID: 27803481 Review. Japanese.
-
Safety, pharmacokinetics, and pharmacodynamics of efzimfotase alfa, a second-generation enzyme replacement therapy: phase 1, dose-escalation study in adults with hypophosphatasia.J Bone Miner Res. 2024 Sep 26;39(10):1412-1423. doi: 10.1093/jbmr/zjae128. J Bone Miner Res. 2024. PMID: 39135540 Free PMC article. Clinical Trial.
Cited by
-
Bone-targeting endogenous secretory receptor for advanced glycation end products rescues rheumatoid arthritis.Mol Med. 2013 Jul 24;19(1):183-94. doi: 10.2119/molmed.2012.00309. Mol Med. 2013. PMID: 23821362 Free PMC article.
-
Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome.Drug Des Devel Ther. 2015 Apr 1;9:1937-53. doi: 10.2147/DDDT.S68562. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25897204 Free PMC article. Review.
-
Selective drug delivery to bone using acidic oligopeptides.J Bone Miner Metab. 2009;27(1):1-8. doi: 10.1007/s00774-008-0004-z. Epub 2008 Nov 19. J Bone Miner Metab. 2009. PMID: 19018455 Review. No abstract available.
-
Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model.Mol Genet Metab. 2012 Sep;107(1-2):161-72. doi: 10.1016/j.ymgme.2012.07.002. Epub 2012 Jul 14. Mol Genet Metab. 2012. PMID: 22902520 Free PMC article.
-
Skeletal Dysplasias: Growing Therapy for Growing Bones.Front Pharmacol. 2017 Mar 6;8:79. doi: 10.3389/fphar.2017.00079. eCollection 2017. Front Pharmacol. 2017. PMID: 28321190 Free PMC article. Review.
References
-
- Fraser D. Hypophosphatasia. Am J Med. 1957;22:730–746. - PubMed
-
- Whyte MP. Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. McGraw-Hill; New York: 2001. pp. 5313–5329.
-
- Silver MM, Vilos GA, Milne KJ. Pulmonary hypophosphatasia in neonatal hypophosphatasia. Pediatr Pathol. 1988;8:483–493. - PubMed
-
- Ali SY. Matrix formation and mineralization in bone. In: Whitehead CC, editor. Bone Biology and Skeletal Disorders. Carfax Publishing Co.; Abingdon, UK: 1992. pp. 19–38.
-
- Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995;314:266–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

