Pseudomonas aeruginosa porin OprF: properties of the channel
- PMID: 16617058
- PMCID: PMC2846715
- DOI: 10.1074/jbc.M600650200
Pseudomonas aeruginosa porin OprF: properties of the channel
Abstract
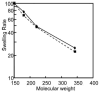
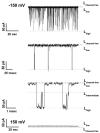
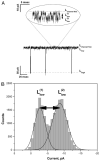
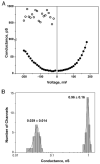
Using ion channel reconstitution in planar lipid bilayers, we examined the channel-forming activity of subfractions of Pseudomonas aeruginosa OprF, which was shown to exist in two different conformations: a minority single domain conformer and a majority two-domain conformer (Sugawara, E., Nestorovich, E. M., Bezrukov, S. M., and Nikaido, H. (2006) J. Biol. Chem. 281, 16220-16229). With the fraction depleted for the single domain conformer, we were unable to detect formation of any channels with well defined conductance levels. With the unfractionated OprF, we saw only rare channel formation. However, with the single domain-enriched fraction of OprF, we observed regular insertion of channels with highly reproducible conductances. Single OprF channels demonstrate rich kinetic behavior exhibiting spontaneous transitions between several subconformations that differ in ionic conductance and radius measured in polymer exclusion experiments. Although we showed that the effective radius of the most conductive conformation exceeds that of the general outer membrane porin of Escherichia coli, OmpF, we also found that a single OprF channel mainly exists in weakly conductive subconformations and switches to the fully open state for a short time only. Therefore, the low permeability of OprF reported earlier may be due to two factors: mainly to the paucity of the single domain conformer in the OprF population and secondly to the predominance of weakly conductive subconformations within the single domain conformer.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

