Disparate thermodynamics governing T cell receptor-MHC-I interactions implicate extrinsic factors in guiding MHC restriction
- PMID: 16617112
- PMCID: PMC1564203
- DOI: 10.1073/pnas.0600743103
Disparate thermodynamics governing T cell receptor-MHC-I interactions implicate extrinsic factors in guiding MHC restriction
Abstract
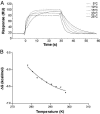
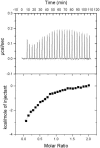
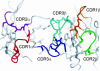
The underlying basis of major histocompatibility complex (MHC) restriction is unclear. Nevertheless, current data suggest that a common thermodynamic signature dictates alphabeta T cell receptor (TcR) ligation. To evaluate whether this thermodynamic signature defines MHC restriction, we have examined the thermodynamic basis of a highly characterized immunodominant TcR interacting with its cognate peptide-MHC-I ligand. Surprisingly, we observed this interaction to be governed by favorable enthalpic and entropic forces, which is in contrast to the prevailing generality, namely, enthalpically driven interactions combined with markedly unfavorable entropic forces. We conclude that extrinsic molecular factors, such as coreceptor ligation, conformational adjustments involved in TcR signaling, or constraints dictated by higher-order arrangement of ligated TcRs, might play a greater role in guiding MHC restriction than appreciated previously.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- van der Merwe P. A., Davis S. J. Annu. Rev. Immunol. 2003;21:659–684. - PubMed
-
- Rudolph M. G., Wilson I. A. Curr. Opin. Immunol. 2002;14:52–65. - PubMed
-
- Ely L. K., Kjer-Nielsen L., McCluskey J., Rossjohn J. IUBMB Life. 2005;57:575–582. - PubMed
-
- Garcia K. C., Adams E. J. Cell. 2005;122:333–336. - PubMed
-
- Wu L. C., Tuot D. S., Lyons D. S., Garcia K. C., Davis M. M. Nature. 2002;418:552–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

