Solution structures of spinach acyl carrier protein with decanoate and stearate
- PMID: 16618110
- PMCID: PMC2533275
- DOI: 10.1021/bi052062d
Solution structures of spinach acyl carrier protein with decanoate and stearate
Abstract
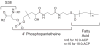
Acyl carrier protein (ACP) is a cofactor in a variety of biosynthetic pathways, including fatty acid metabolism. Thus, it is of interest to determine structures of physiologically relevant ACP-fatty acid complexes. We report here the NMR solution structures of spinach ACP with decanoate (10:0-ACP) and stearate (18:0-ACP) attached to the 4'-phosphopantetheine prosthetic group. The protein in the fatty acid complexes adopts a single conformer, unlike apo- and holo-ACP, which interconvert in solution between two major conformers. The protein component of both 10:0- and 18:0-ACP adopts the four-helix bundle topology characteristic of ACP, and a fatty acid binding cavity was identified in both structures. Portions of the protein close in space to the fatty acid and the 4'-phosphopantetheine were identified using filtered/edited NOESY experiments. A docking protocol was used to generate protein structures containing bound fatty acid for 10:0- and 18:0-ACP. In both cases, the predominant structure contained fatty acid bound down the center of the helical bundle, in agreement with the location of the fatty acid binding pockets. These structures demonstrate the conformational flexibility of spinach ACP and suggest how the protein changes to accommodate its myriad binding partners.
Figures
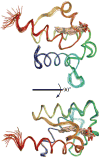
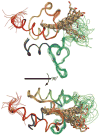


References
-
- Issartel J, Koronakis V, Hughes C. Activation of Escherichia-coli Prohaemolysin to the Mature Toxin by Acyl Carrier Protein-Dependent Fatty Acylation. Nature. 1991;351:759–761. - PubMed
-
- Rumley M, Therisod H, Weissborn A, Kennedy E. Mechanisms of Regulation of the Biosynthesis of Membrane-Derived Oligosaccharides in Escherichia coli. J Biol Chem. 1992;267:11806–11810. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

