A faster migrating variant masquerades as NICD when performing in vitro gamma-secretase assays with bacterially expressed Notch substrates
- PMID: 16618124
- PMCID: PMC2546868
- DOI: 10.1021/bi052228a
A faster migrating variant masquerades as NICD when performing in vitro gamma-secretase assays with bacterially expressed Notch substrates
Abstract
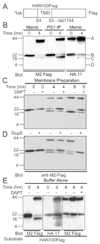
Intramembrane proteolysis is a new and rapidly growing field. In vitro assays utilizing recombinant substrates for gamma-secretase, an intramembrane-cleaving enzyme, are critically important in order to characterize the biochemical properties of this unusual enzyme. Several recombinant Notch proteins of varying length are commonly used as in vitro substrates for CHAPSO-solubilized gamma-secretase. Here we report that several recombinant Notch constructs undergo limited or no proteolysis in vitro. Instead, upon incubation with or without gamma-secretase, variants of the intact protein migrate during SDS-PAGE at the location expected for the gamma-secretase specific cleavage products. In addition, we show that addition of aspartyl- and gamma-secretase specific protease inhibitors are able to retard the formation of these variants independent of gamma-secretase, which could lead to the erroneous conclusion that Notch cleavage by solubilized gamma-secretase was achieved in vitro even when no proteolysis occurred. In contrast, substrates produced in mammalian or insect cells are cleaved efficiently in vitro. These observations suggest that in vitro studies reliant on recombinant, bacterially produced Notch TMD should be performed with the inclusion of additional controls able to differentiate between actual cleavage and this potential artifact.
Figures




Similar articles
-
Analysis of transmembrane domain mutants is consistent with sequential cleavage of Notch by gamma-secretase.J Neurochem. 2006 Jan;96(1):228-35. doi: 10.1111/j.1471-4159.2005.03547.x. Epub 2005 Nov 21. J Neurochem. 2006. PMID: 16300632
-
Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s).Biochemistry. 2003 Jan 14;42(1):137-44. doi: 10.1021/bi026888g. Biochemistry. 2003. PMID: 12515548
-
The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments.J Biol Chem. 2003 Sep 5;278(36):34427-37. doi: 10.1074/jbc.M302659200. Epub 2003 Jun 25. J Biol Chem. 2003. PMID: 12826675
-
Gamma-secretase inhibition.Biochem Soc Trans. 2002 Aug;30(4):534-7. doi: 10.1042/bst0300534. Biochem Soc Trans. 2002. PMID: 12196131 Review.
-
γ-Secretase-regulated signaling typified by Notch signaling in the immune system.Curr Stem Cell Res Ther. 2013 Sep;8(5):341-56. doi: 10.2174/1574888x113089990054. Curr Stem Cell Res Ther. 2013. PMID: 23957936 Review.
Cited by
-
Role of co-repressor genomic landscapes in shaping the Notch response.PLoS Genet. 2017 Nov 20;13(11):e1007096. doi: 10.1371/journal.pgen.1007096. eCollection 2017 Nov. PLoS Genet. 2017. PMID: 29155828 Free PMC article.
References
-
- Haass C, Steiner H. Alzheimer disease gamma-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002;12:556–562. - PubMed
-
- Weihofen A, Martoglio B. Intramembrane-cleaving proteases: controlled liberation of proteins and bioactive peptides. Trends Cell Biol. 2003;13:71–78. - PubMed
-
- Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Current Opinion in Neurobiology. 2004;14:379–383. - PubMed
-
- Wolfe MS. Presenilin and gamma-secretase: structure meets function. J Neurochem. 2001;76:1615–1620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

