Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells
- PMID: 16618809
- PMCID: PMC2063815
- DOI: 10.1083/jcb.200506163
Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells
Abstract
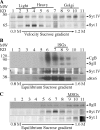
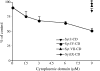
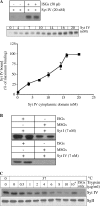
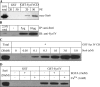
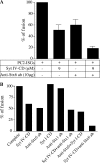
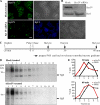
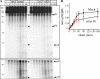
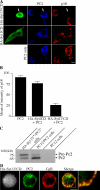
In neuroendocrine PC12 cells, immature secretory granules (ISGs) mature through homotypic fusion and membrane remodeling. We present evidence that the ISG-localized synaptotagmin IV (Syt IV) is involved in ISG maturation. Using an in vitro homotypic fusion assay, we show that the cytoplasmic domain (CD) of Syt IV, but not of Syt I, VII, or IX, inhibits ISG homotypic fusion. Moreover, Syt IV CD binds specifically to ISGs and not to mature secretory granules (MSGs), and Syt IV binds to syntaxin 6, a SNARE protein that is involved in ISG maturation. ISG homotypic fusion was inhibited in vivo by small interfering RNA-mediated depletion of Syt IV. Furthermore, the Syt IV CD, as well as Syt IV depletion, reduces secretogranin II (SgII) processing by prohormone convertase 2 (PC2). PC2 is found mostly in the proform, suggesting that activation of PC2 is also inhibited. Granule formation, and the sorting of SgII and PC2 from the trans-Golgi network into ISGs and MSGs, however, is not affected. We conclude that Syt IV is an essential component for secretory granule maturation.
Figures

References
-
- Austin, C., I. Hinners, and S.A. Tooze. 2000. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 275:21862–21869. - PubMed
-
- Bai, J., and E.R. Chapman. 2004. The C2 domains of synaptotagmin–partners in exocytosis. Trends Biochem. Sci. 29:143–151. - PubMed
-
- Bennett, M.K., N. Calakos, and R.H. Scheller. 1992. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 257:255–259. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

