Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade
- PMID: 16618925
- PMCID: PMC1458950
- DOI: 10.1073/pnas.0510645103
Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade
Abstract
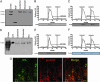
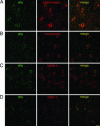
In this report, we provide direct demonstration that the neurotrophin nerve growth factor (NGF) is released in the extracellular space in an activity-dependent manner in its precursor form (proNGF) and that it is in this compartment that its maturation and degradation takes place because of the coordinated release and the action of proenzymes and enzyme regulators. This converting protease cascade and its endogenous regulators (including tissue plasminogen activator, plasminogen, neuroserpin, precursor matrix metalloproteinase 9, and tissue inhibitor metalloproteinase 1) are colocalized in neurons of the cerebral cortex and released upon neuronal stimulation. We also provide evidence that this mechanism operates in in vivo conditions, as the CNS application of inhibitors of converting and degrading enzymes lead to dramatic alterations in the tissue levels of either precursor NGF or mature NGF. Pathological alterations of this cascade in the CNS might cause or contribute to a lack of proper neuronal trophic support in conditions such as cerebral ischemia, seizure and Alzheimer's disease or, conversely, to excessive local production of neurotrophins as reported in inflammatory arthritis pain.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
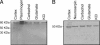
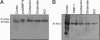



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

