The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment
- PMID: 16619026
- PMCID: PMC1456945
- DOI: 10.1038/sj.emboj.7601088
The Hrd1p ligase complex forms a linchpin between ER-lumenal substrate selection and Cdc48p recruitment
Abstract
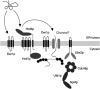
Misfolded proteins of the endoplasmic reticulum (ER) are targeted to the cytoplasm for proteasomal degradation. Key components of this process are ER membrane-bound ubiquitin ligases. These ligases associate with the cytoplasmic AAA-ATPase Cdc48p/p97, which is thought to support the release of malfolded proteins from the ER. Here, we characterize a yeast protein complex containing the ubiquitin ligase Hrd1p and the ER membrane proteins Hrd3p and Der1p. Hrd3p binds malfolded proteins in the ER lumen enabling their delivery to downstream components. Therefore, we propose that Hrd3p acts as a substrate recruitment factor for the Hrd1p ligase complex. Hrd3p function is also required for the association of Cdc48p with Hrd1p. Moreover, our data demonstrate that recruitment of Cdc48p depends on substrate processing by the Hrd1p ligase complex. Thus, the Hrd1p ligase complex unites substrate selection in the ER lumen and polyubiquitination in the cytoplasm and links these processes to the release of ER proteins via the Cdc48p complex.
Figures







References
-
- Ausubel FM (eds) (1993–2004) Current Protocols in Molecular Biology. New York: John Wiley & Sons, Inc.
-
- Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY (2001a) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol 3: 24–29 - PubMed
-
- Biederer T, Volkwein C, Sommer T (1997) Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278: 1806–1809 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

