Role of SGT1 in resistance protein accumulation in plant immunity
- PMID: 16619029
- PMCID: PMC1456927
- DOI: 10.1038/sj.emboj.7601084
Role of SGT1 in resistance protein accumulation in plant immunity
Abstract
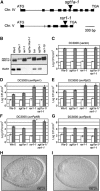
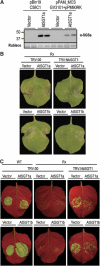
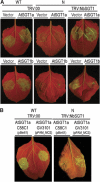
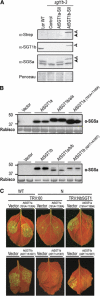
A highly conserved eukaryotic protein SGT1 binds specifically to the molecular chaperone, HSP90. In plants, SGT1 positively regulates disease resistance conferred by many Resistance (R) proteins and developmental responses to the phytohormone, auxin. We show that silencing of SGT1 in Nicotiana benthamiana causes a reduction in steady-state levels of the R protein, Rx. These data support a role of SGT1 in R protein accumulation, possibly at the level of complex assembly. In Arabidopsis, two SGT1 proteins, AtSGT1a and AtSGT1b, are functionally redundant early in development. AtSGT1a and AtSGT1b are induced in leaves upon infection and either protein can function in resistance once a certain level is attained, depending on the R protein tested. In unchallenged tissues, steady-state AtSGT1b levels are at least four times greater than AtSGT1a. While the respective tetratricopeptide repeat (TPR) domains of SGT1a and SGT1b control protein accumulation, they are dispensable for intrinsic functions of SGT1 in resistance and auxin responses.
Figures





References
-
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JD, Parker JE (2002) Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295: 2077–2080 - PubMed
-
- Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K, Schulze-Lefert P (2002) The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295: 2073–2076 - PubMed
-
- Belkhadir Y, Subramaniam R, Dangl JL (2004) Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol 7: 391–399 - PubMed
-
- Bieri S, Mauch S, Shen QH, Peart J, Devoto A, Casais C, Ceron F, Schulze S, Steinbiss HH, Shirasu K, Schulze-Lefert P (2004) RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell 16: 3480–3495 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

