Microstimulation of frontal cortex can reorder a remembered spatial sequence
- PMID: 16620152
- PMCID: PMC1440931
- DOI: 10.1371/journal.pbio.0040134
Microstimulation of frontal cortex can reorder a remembered spatial sequence
Abstract
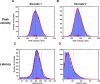
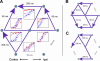
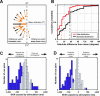
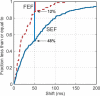
Complex goal-directed behaviors extend over time and thus depend on the ability to serially order memories and assemble compound, temporally coordinated movements. Theories of sequential processing range from simple associative chaining to hierarchical models in which order is encoded explicitly and separately from sequence components. To examine how short-term memory and planning for sequences might be coded, we used microstimulation to perturb neural activity in the supplementary eye field (SEF) while animals held a sequence of two cued locations in memory over a short delay. We found that stimulation affected the order in which animals saccaded to the locations, but not the memory for which locations were cued. These results imply that memory for sequential order can be dissociated from that of its components. Furthermore, stimulation of the SEF appeared to bias sequence endpoints to converge toward a location in contralateral space, suggesting that this area encodes sequences in terms of their endpoints rather than their individual components.
Figures




Comment in
-
Evidence for the encoding of a motion's goal in the monkey brain.PLoS Biol. 2006 May;4(5):e169. doi: 10.1371/journal.pbio.0040169. Epub 2006 Apr 25. PLoS Biol. 2006. PMID: 20076576 Free PMC article. No abstract available.
References
-
- Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior. New York: Wiley; 1951. pp. 112–136.
-
- Abeles M. Corticonics: Neural circuits of the cerebral cortex. Cambridge: Cambridge University Press; 1991. 280 pp.
-
- Berns GS, Sejnowski TJ. A computational model of how the basal ganglia produce sequences. J Cogn Neurosci. 1998;10:108–121. - PubMed
-
- Nakahara H, Doya K, Hikosaka O. Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences: A computational approach. J Cogn Neurosci. 2001;13:626–647. - PubMed
-
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

