Immunization with SARS-CoV S DNA vaccine generates memory CD4+ and CD8+ T cell immune responses
- PMID: 16621188
- PMCID: PMC7115633
- DOI: 10.1016/j.vaccine.2006.03.058
Immunization with SARS-CoV S DNA vaccine generates memory CD4+ and CD8+ T cell immune responses
Abstract
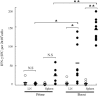
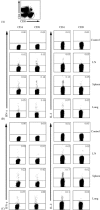
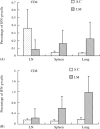
An effective vaccine for severe acute respiratory syndrome (SARS) will probably require the generation and maintenance of both humoral and cellular immune responses. It has been reported that after natural infection in humans and immunization in animals with SARS-CoV vaccine, antibody is produced and persistent for a long period of time. In the present study, mice were immunized i.m. with SARS-CoV S DNA vaccine, and three different methods (ELISA, ELISPOT and FACS) were used to evaluate the immune responses when the cells were stimulated in vitro with a pool of peptides overlapping entire SARS spike protein. The results show that prime-immunization with SARS-CoV S DNA vaccine can induce both CD4(+) and CD8(+) T cell responses. Boosting with the same vaccine enhances CD4(+) and CD8(+) T cell responses in both lymphoid and nonlymphoid organs and were persistent over two months. The SARS-CoV S-specific CD4(+) and CD8(+) T cells were CD62L(-), a marker for memory cells, and -30 to 50% of the cells expressed IL-7Ralpha (CD127), a marker for the capacity of effector cells to develop into memory cells. In addition, immunization with the DNA vaccine elicited high levels of antibody production. Taken together, these data demonstrate that immunization with SARS-CoV S DNA vaccine can generate antigen-specific humoral and cellular immune responses that may contribute to long-term protection.
Figures



Similar articles
-
Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses.Vaccine. 2007 Sep 28;25(39-40):6981-91. doi: 10.1016/j.vaccine.2007.06.047. Epub 2007 Jul 16. Vaccine. 2007. PMID: 17709158 Free PMC article.
-
CD8+ T cell response in HLA-A*0201 transgenic mice is elicited by epitopes from SARS-CoV S protein.Vaccine. 2010 Sep 24;28(41):6666-74. doi: 10.1016/j.vaccine.2010.08.013. Epub 2010 Aug 13. Vaccine. 2010. PMID: 20709007 Free PMC article.
-
Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection.J Virol. 2014 Oct;88(19):11034-44. doi: 10.1128/JVI.01505-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056892 Free PMC article.
-
SARS Immunity and Vaccination.Cell Mol Immunol. 2004 Jun;1(3):193-8. Cell Mol Immunol. 2004. PMID: 16219167 Review.
-
T cell-mediated immune response to respiratory coronaviruses.Immunol Res. 2014 Aug;59(1-3):118-28. doi: 10.1007/s12026-014-8534-z. Immunol Res. 2014. PMID: 24845462 Free PMC article. Review.
Cited by
-
Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses.Mol Immunol. 2008 Feb;45(4):868-75. doi: 10.1016/j.molimm.2007.08.010. Epub 2007 Oct 1. Mol Immunol. 2008. PMID: 17905435 Free PMC article.
-
Immunogenicity and safety of a COVID-19 DNA vaccine in healthy adults and elderly: A randomized, observer-blind, placebo-controlled phase 2 trial.Hum Vaccin Immunother. 2025 Dec;21(1):2448405. doi: 10.1080/21645515.2024.2448405. Epub 2025 Jan 26. Hum Vaccin Immunother. 2025. PMID: 39865693 Free PMC article. Clinical Trial.
-
Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: implications for severe acute respiratory syndrome.J Virol. 2009 Sep;83(18):9258-72. doi: 10.1128/JVI.00355-09. Epub 2009 Jul 1. J Virol. 2009. PMID: 19570864 Free PMC article.
-
T cell epitope specificity and pathogenesis of mouse hepatitis virus-1-induced disease in susceptible and resistant hosts.J Immunol. 2010 Jul 15;185(2):1132-41. doi: 10.4049/jimmunol.0902749. Epub 2010 Jun 16. J Immunol. 2010. PMID: 20554960 Free PMC article.
-
Identification of SARS-CoV-2 Nucleocapsid and Spike T-Cell Epitopes for Assessing T-Cell Immunity.J Virol. 2021 Feb 24;95(6):e02002-20. doi: 10.1128/JVI.02002-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33443088 Free PMC article.
References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. - PubMed
-
- Peiris J.S.M., Yuen K.Y., Osterhaus A.D.M.E., Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

