Mutation of waaC, encoding heptosyltransferase I in Campylobacter jejuni 81-176, affects the structure of both lipooligosaccharide and capsular carbohydrate
- PMID: 16621820
- PMCID: PMC1447440
- DOI: 10.1128/JB.188.9.3273-3279.2006
Mutation of waaC, encoding heptosyltransferase I in Campylobacter jejuni 81-176, affects the structure of both lipooligosaccharide and capsular carbohydrate
Abstract
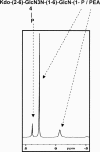
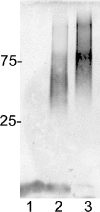
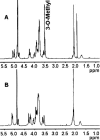
Campylobacter jejuni 81-176 lipooligosaccharide (LOS) is composed of two covalently linked domains: lipid A, a hydrophobic anchor, and a nonrepeating core oligosaccharide, consisting of an inner and outer core region. We report the isolation and characterization of the deepest rough C. jejuni 81-176 mutant by insertional mutagenesis into the waaC gene, encoding heptosyltransferase I that catalyzes the transfer of the first L-glycero-D-manno-heptose residue to 3-deoxy-D-manno-octulosonic residue (Kdo)-lipid A. Tricine gel electrophoresis, followed by silver staining, showed that site-specific mutation in the waaC gene resulted in the expression of a severely truncated LOS compared to wild-type strain 81-176. Gas-liquid chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy showed that the waaC LOS species lacked all sugars distal to Kdo-lipid A. Parallel structural studies of the capsular polysaccharides of the wild-type strain 81-176 and waaC mutant revealed loss of the 3-O-methyl group in the waaC mutant. Complementation of the C. jejuni mutant by insertion of the wild-type C. jejuni waaC gene into a chromosomal locus resulted in LOS and capsular structures identical to those expressed in the parent strain. We also report here the presence of O-methyl phosphoramidate in wild-type strain 81-176 capsular polysaccharide.
Figures



References
-
- Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. - PubMed
-
- Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. - PubMed
-
- Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209-217.
-
- Dubois, M., K. Giles, J. Hamilton, P. Rebers, and F. Smith. 1956. Colometric method for determination of sugars and related substances. Anal. Chem. 28:350-356.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

