Biochemical bases of type IV chromatic adaptation in marine Synechococcus spp
- PMID: 16621829
- PMCID: PMC1447437
- DOI: 10.1128/JB.188.9.3345-3356.2006
Biochemical bases of type IV chromatic adaptation in marine Synechococcus spp
Abstract
Chromatic adaptation (CA) in cyanobacteria has provided a model system for the study of the environmental control of photophysiology for several decades. All forms of CA that have been examined so far (types II and III) involve changes in the relative contents of phycoerythrin (PE) and/or phycocyanin when cells are shifted from red to green light and vice versa. However, the chromophore compositions of these polypeptides are not altered. Some marine Synechococcus species strains, which possess two PE forms (PEI and PEII), carry out another type of CA (type IV), occurring during shifts from blue to green or white light. Two chromatically adapting strains of marine Synechococcus recently isolated from the Gulf of Mexico were utilized to elucidate the mechanism of type IV CA. During this process, no change in the relative contents of PEI and PEII was observed. Instead, the ratio of the two chromophores bound to PEII, phycourobilin and phycoerythrobilin, is high under blue light and low under white light. Mass spectroscopy analyses of isolated PEII alpha- and beta-subunits show that there is a single PEII protein type under all light climates. The CA process seems to specifically affect the chromophorylation of the PEII (and possibly PEI) alpha chain. We propose a likely process for type IV CA, which involves the enzymatic activity of one or several phycobilin lyases and/or lyase-isomerases differentially controlled by the ambient light quality. Phylogenetic analyses based on the 16S rRNA gene confirm that type IV CA is not limited to a single clade of marine Synechococcus.
Figures

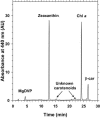





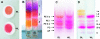


References
-
- Anderson, L. K., M. C. Rayner, and F. A. Eiserling. 1984. Ultra-violet mutagenesis of Synechocystis sp. 6701: mutations in chromatic adaptation and phycobilisome assembly. Arch. Microbiol. 138:237-243.
-
- Campbell, L., and R. Iturriaga. 1988. Identification of Synechococcus spp. in the Sargasso Sea by immunofluorescence and fluorescence excitation spectroscopy performed on individual cells. Limnol. Oceanogr. 33:1196-1201.
-
- Conley, P. B., P. G. Lemaux, and A. Grossman. 1988. Molecular characterization and evolution of sequences encoding light-harvesting components in the chromatically adapting cyanobacterium Fremyella diplosiphon. J. Mol. Biol. 199:447-465. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

