Heme and a five-amino-acid hemophore region form the bipartite stimulus triggering the has signaling cascade
- PMID: 16621830
- PMCID: PMC1447456
- DOI: 10.1128/JB.188.9.3357-3364.2006
Heme and a five-amino-acid hemophore region form the bipartite stimulus triggering the has signaling cascade
Abstract
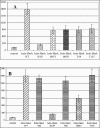
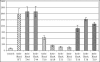
Bacterial cells sense the extracellular environment and adapt to that environment by activating gene regulation circuits, often by means of signaling molecules. The Serratia marcescens hemophore is a signaling molecule that acts as an extracellular heme-scavenging protein. The heme-loaded hemophore interacts with its cognate receptor (HasR), triggering transmembrane signaling and turning on transcription of hemophore-dependent heme uptake genes. We investigated the features of the holo-hemophore, the only HasR ligand known to act as an inducer. We used a hemophore mutant that does not deliver its heme and a HasR mutant that does not bind heme, and we showed that heme transfer from the hemophore to the receptor is necessary for induction. Using a hemophore mutant that does not bind heme and that blocks heme transport, we demonstrated that two molecules that do not interact (heme and the mutant hemophore) may nonetheless induce this system. These findings suggest that hemophore-mediated induction and heme transport involve different mechanisms. The hemophore region important for induction was precisely localized to amino acids 50 to 55, which lie in one of the two HasR-binding hemophore regions. This bipartite stimulus probably corresponds to a physiological process because heme is transferred to the receptor before apo-hemophore release. This bipartite regulation mechanism may allow the bacterium to adjust its heme transport mechanism to the perceived environmental heme concentration.
Figures




Similar articles
-
Activities of the Serratia marcescens heme receptor HasR and isolated plug and beta-barrel domains: the beta-barrel forms a heme-specific channel.J Bacteriol. 2005 Jul;187(13):4637-45. doi: 10.1128/JB.187.13.4637-4645.2005. J Bacteriol. 2005. PMID: 15968075 Free PMC article.
-
Comparative analysis of structural and dynamic properties of the loaded and unloaded hemophore HasA: functional implications.J Mol Biol. 2008 Feb 15;376(2):517-25. doi: 10.1016/j.jmb.2007.11.072. Epub 2007 Dec 4. J Mol Biol. 2008. PMID: 18164722
-
Heme acquisition by hemophores.Biometals. 2007 Jun;20(3-4):603-13. doi: 10.1007/s10534-006-9050-y. Epub 2007 Feb 1. Biometals. 2007. PMID: 17268821 Review.
-
Binding of HasA by its transmembrane receptor HasR follows a conformational funnel mechanism.Eur Biophys J. 2020 Jan;49(1):39-57. doi: 10.1007/s00249-019-01411-1. Epub 2019 Dec 4. Eur Biophys J. 2020. PMID: 31802151 Free PMC article.
-
Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores.Curr Opin Microbiol. 2000 Apr;3(2):215-20. doi: 10.1016/s1369-5274(00)00078-3. Curr Opin Microbiol. 2000. PMID: 10744995 Review.
Cited by
-
An Exposed Outer Membrane Hemin-Binding Protein Facilitates Hemin Transport by a TonB-Dependent Receptor in Riemerella anatipestifer.Appl Environ Microbiol. 2021 Jul 13;87(15):e0036721. doi: 10.1128/AEM.00367-21. Epub 2021 Jul 13. Appl Environ Microbiol. 2021. PMID: 33990314 Free PMC article.
-
Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex.Proc Natl Acad Sci U S A. 2009 Jan 27;106(4):1045-50. doi: 10.1073/pnas.0809406106. Epub 2009 Jan 14. Proc Natl Acad Sci U S A. 2009. PMID: 19144921 Free PMC article.
-
Bacterial heme-transport proteins and their heme-coordination modes.Arch Biochem Biophys. 2009 Jan 1;481(1):1-15. doi: 10.1016/j.abb.2008.10.013. Epub 2008 Oct 17. Arch Biochem Biophys. 2009. PMID: 18977196 Free PMC article. Review.
-
Extracellular haem utilization by the opportunistic pathogen Pseudomonas aeruginosa and its role in virulence and pathogenesis.Adv Microb Physiol. 2021;79:89-132. doi: 10.1016/bs.ampbs.2021.07.004. Epub 2021 Aug 13. Adv Microb Physiol. 2021. PMID: 34836613 Free PMC article.
-
Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut.Life (Basel). 2025 Jun 13;15(6):950. doi: 10.3390/life15060950. Life (Basel). 2025. PMID: 40566603 Free PMC article.
References
-
- Arnoux, P., R. Haser, N. Izadi, A. Lecroisey, M. Delepierre, C. Wandersman, and M. Czjzek. 1999. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat. Struct. Biol. 6:516-520. - PubMed
-
- Bashyam, M. D., and S. E. Hasnain. 2004. The extracytoplasmic function sigma factors: role in bacterial pathogenesis. Infect. Genet. Evol. 4:301-308. - PubMed
-
- Beare, P. A., R. J. For, L. W. Martin, and I. L. Lamont. 2003. Siderophore-mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol. Microbiol. 47:195-207. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

