The Src kinase Lyn is required for CCR5 signaling in response to MIP-1beta and R5 HIV-1 gp120 in human macrophages
- PMID: 16621960
- PMCID: PMC1895866
- DOI: 10.1182/blood-2005-12-012815
The Src kinase Lyn is required for CCR5 signaling in response to MIP-1beta and R5 HIV-1 gp120 in human macrophages
Abstract
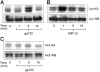
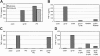
CCR5 is a receptor for several beta chemokines and the entry coreceptor used by macrophage-tropic (R5) strains of HIV-1. In addition to supporting viral entry, CCR5 ligation by the HIV-1 envelope glycoprotein 120 (gp120) can activate intracellular signals in macrophages and trigger inflammatory mediator release. Using a combination of in vitro kinase assay, Western blotting for phospho-specific proteins, pharmacologic inhibition, CCR5 knockout (CCR5Delta32) cells, and kinase-specific blocking peptide, we show for the first time that signaling through CCR5 in primary human macrophages is linked to the Src kinase Lyn. Stimulation of human monocyte-derived macrophages with either HIV-1 gp120 or MIP-1beta results in the CCR5-mediated activation of Lyn and the concomitant Lyn-dependent activation of the mitogen-activated protein (MAP) kinase ERK-1/2. Furthermore, activation of the CCR5/Lyn/ERK-1/2 pathway is responsible for gp120-triggered production of TNF-alpha by macrophages, which is believed to contribute to HIV-1 pathogenesis. Thus, Lyn kinase may play an important role both in normal CCR5 function in macrophages and in AIDS pathogenesis in syndromes such as AIDS dementia where HIV-1 gp120 contributes to inappropriate macrophage activation, mediator production, and secondary injury.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

