Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome
- PMID: 16621965
- PMCID: PMC1895874
- DOI: 10.1182/blood-2005-10-007252
Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome
Abstract
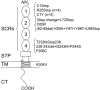
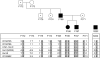
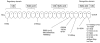
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy with manifestations of hemolytic anemia, thrombocytopenia, and renal impairment. Genetic studies have shown that mutations in complement regulatory proteins predispose to non-Shiga toxin-associated HUS (non-Stx-HUS). We undertook genetic analysis on membrane cofactor protein (MCP), complement factor H (CFH), and factor I (IF) in 156 patients with non-Stx-HUS. Fourteen, 11, and 5 new mutational events were found in MCP, CFH, and IF, respectively. Mutation frequencies were 12.8%, 30.1%, and 4.5% for MCP, CFH, and IF, respectively. MCP mutations resulted in either reduced protein expression or impaired C3b binding capability. MCP-mutated patients had a better prognosis than CFH-mutated and nonmutated patients. In MCP-mutated patients, plasma treatment did not impact the outcome significantly: remission was achieved in around 90% of both plasma-treated and plasma-untreated acute episodes. Kidney transplantation outcome was favorable in patients with MCP mutations, whereas the outcome was poor in patients with CFH and IF mutations due to disease recurrence. This study documents that the presentation, the response to therapy, and the outcome of the disease are influenced by the genotype. Hopefully this will translate into improved management and therapy of patients and will provide the way to design tailored treatments.
Figures
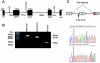


References
-
- Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60: 831-846. - PubMed
-
- Boyce TG, Swerdlow DL, Griffin PM. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333: 364-368. - PubMed
-
- Kaplan BS, Meyers KE, Schulman SL. The pathogenesis and treatment of hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9: 1126-1133. - PubMed
-
- Schieppati A, Ruggenenti P, Cornejo RP, et al. Renal function at hospital admission as a prognostic factor in adult hemolytic uremic syndrome: The Italian Registry of Haemolytic Uremic Syndrome. J Am Soc Nephrol. 1992;2: 1640-1644. - PubMed
-
- Taylor CM, Chua C, Howie AJ, Risdon RA. Clinico-pathological findings in diarrhoea-negative haemolytic uraemic syndrome. Pediatr Nephrol. 2004;19: 419-425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

