The hemoglobin receptor protein of porphyromonas gingivalis inhibits receptor activator NF-kappaB ligand-induced osteoclastogenesis from bone marrow macrophages
- PMID: 16622189
- PMCID: PMC1459701
- DOI: 10.1128/IAI.74.5.2544-2551.2006
The hemoglobin receptor protein of porphyromonas gingivalis inhibits receptor activator NF-kappaB ligand-induced osteoclastogenesis from bone marrow macrophages
Abstract
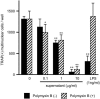
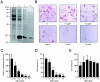
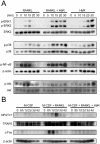
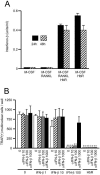
Extracellular proteinaceous factors of Porphyromonas gingivalis, a periodontal pathogen, that influence receptor activator of nuclear factor-kappaB (NF-kappaB) ligand (RANKL)-induced osteoclastogenesis from bone marrow macrophages were investigated. The culture supernatant of P. gingivalis had the ability to inhibit RANKL-induced in vitro osteoclastogenesis. A major protein of the culture supernatant, hemoglobin receptor protein (HbR), suppressed RANKL-induced osteoclastogenesis in a dose-dependent fashion. HbR markedly inhibited RANKL-induced osteoclastogenesis when present in the culture for the first 24 h after addition of RANKL, whereas no significant inhibition was observed when HbR was added after 24 h or later, implying that HbR might interfere with only the initial stage of RANKL-mediated differentiation. HbR tightly bound to bone marrow macrophages and had the ability to induce phosphorylation of ERK, p38, NF-kappaB, and Akt. RANKL-induced phosphorylation of ERK, p38, and NF-kappaB was not suppressed by HbR, but that of Akt was markedly suppressed. HbR inhibited RANKL-mediated induction of c-Fos and NFATc1. HbR could induce beta interferon (IFN-beta) from bone marrow macrophages, but the induction level of IFN-beta might not be sufficient to suppress RANKL-mediated osteoclastogenesis, implying presence of an IFN-beta-independent pathway in HbR-mediated inhibition of osteoclastogenesis. Since rapid and extensive destruction of the alveolar bone causes tooth loss, resulting in loss of the gingival crevice that is an anatomical niche for periodontal pathogens such as P. gingivalis, the suppressive effect of HbR on osteoclastogenesis may help the microorganism exist long in the niche.
Figures



Similar articles
-
12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits osteoclastogenesis by suppressing RANKL-induced NF-kappaB activation.J Bone Miner Res. 2003 Dec;18(12):2159-68. doi: 10.1359/jbmr.2003.18.12.2159. J Bone Miner Res. 2003. PMID: 14672351
-
Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis.J Immunol. 2004 May 15;172(10):5940-7. doi: 10.4049/jimmunol.172.10.5940. J Immunol. 2004. PMID: 15128775
-
Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1.J Bone Miner Res. 2005 Apr;20(4):653-62. doi: 10.1359/JBMR.041213. Epub 2004 Dec 6. J Bone Miner Res. 2005. PMID: 15765185
-
Prostaglandin E(2) is a main mediator in receptor activator of nuclear factor-kappaB ligand-dependent osteoclastogenesis induced by Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii.J Periodontol. 2005 May;76(5):813-20. doi: 10.1902/jop.2005.76.5.813. J Periodontol. 2005. PMID: 15898943
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
Cited by
-
Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms.Periodontol 2000. 2010 Feb;52(1):94-116. doi: 10.1111/j.1600-0757.2009.00329.x. Periodontol 2000. 2010. PMID: 20017798 Free PMC article. Review. No abstract available.
-
Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis.Front Cell Infect Microbiol. 2013 Dec 19;3:101. doi: 10.3389/fcimb.2013.00101. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24392356 Free PMC article. Review.
-
Hemoglobin receptor protein from Porphyromonas gingivalis induces interleukin-8 production in human gingival epithelial cells through stimulation of the mitogen-activated protein kinase and NF-κB signal transduction pathways.Infect Immun. 2014 Jan;82(1):202-11. doi: 10.1128/IAI.01140-12. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126532 Free PMC article.
-
Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases.Jpn Dent Sci Rev. 2017 Nov;53(4):134-140. doi: 10.1016/j.jdsr.2017.06.001. Epub 2017 Aug 9. Jpn Dent Sci Rev. 2017. PMID: 29201258 Free PMC article. Review.
-
Oral Mucosal Epithelial Cells.Front Immunol. 2019 Feb 14;10:208. doi: 10.3389/fimmu.2019.00208. eCollection 2019. Front Immunol. 2019. PMID: 30837987 Free PMC article. Review.
References
-
- Boyle, W. J., W. S. Simonet, and D. L. Lacey. 2003. Osteoclast differentiation and activation. Nature 423:337-342. - PubMed
-
- Choi, B. K., S. Y. Moon, J. H. Cha, K. W. Kim, and Y. J. Yoo. 2005. Prostaglandin E2 is a main mediator in receptor activator of nuclear factor-κB ligand-dependent osteoclastogenesis induced by Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii. J. Periodontol. 76:813-820. - PubMed
-
- Curtis, M. A., H. K. Kuramitsu, M. Lantz, F. L. Macrina, K. Nakayama, J. Potempa, E. C. Reynolds, and J. Aduse-Opoku. 1999. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J. Periodontal Res. 34:464-472. - PubMed
-
- DeCarlo, A. A., M. Nadkarni, M. Paramaesvaran, P. W. Yun, C. A. Collyer, and N. Hunter. 2004. Serum antibodies against the hemoglobin-binding domain (HA2) of Porphyromonas gingivalis. J. Periodontal Res. 39:228-235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

