The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa
- PMID: 16622193
- PMCID: PMC1459711
- DOI: 10.1128/IAI.74.5.2578-2586.2006
The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa
Abstract
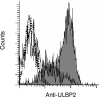
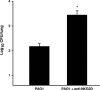
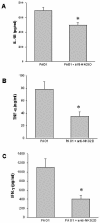
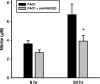
The NKG2D-activating receptor is expressed on cytotoxic lymphocytes and interacts with ligands expressed on the surface of cells stressed by pathogenic and nonpathogenic stimuli. In this study, we investigated the physiologic importance of NKG2D receptor-ligand interactions in response to acute pulmonary Pseudomonas aeruginosa infection. P. aeruginosa infection increased the expression of mouse NKG2D ligands (Rae1) in airway epithelial cells and alveolar macrophages in vivo and also increased the cell surface expression of human NKG2D ligands (ULBP2) on airway epithelial cells in vitro. NKG2D receptor blockade with a specific monoclonal antibody inhibited the pulmonary clearance of P. aeruginosa. NKG2D receptor blockade also resulted in decreased production of Th1 cytokines and nitric oxide in the lungs of P. aeruginosa-infected mice. Additionally, NKG2D receptor blockade reduced the epithelial cell sloughing that accompanies P. aeruginosa infection. Macrophage phagocytosis and bronchoalveolar lavage cellularity were not different in P. aeruginosa-infected mice with and without NKG2D receptor blockade. These results demonstrate the importance of NKG2D-mediated immune activation in the clearance of acute bacterial infection and suggest that epithelial cell-lymphocyte interactions mediate pulmonary cytokine production, epithelial cell integrity, and bacterial clearance.
Figures




References
-
- Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jornvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. - PubMed
-
- Bacon, L., R. A. Eagle, M. Meyer, N. Easom, N. T. Young, and J. Trowsdale. 2004. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J. Immunol. 173:1078-1084. - PubMed
-
- Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727-729. - PubMed
-
- Calum, H., C. Moser, P. O. Jensen, R. Shirai, and N. Hoiby. 2003. Cytokine and surface receptor diversity of NK cells in resistant C3H/HeN and susceptible BALB/c mice with chronic Pseudomonas aeruginosa lung infection. APMIS 111:891-897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

