Outer membrane protein A of Escherichia coli O157:H7 stimulates dendritic cell activation
- PMID: 16622204
- PMCID: PMC1459721
- DOI: 10.1128/IAI.74.5.2676-2685.2006
Outer membrane protein A of Escherichia coli O157:H7 stimulates dendritic cell activation
Abstract
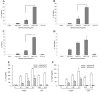
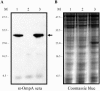
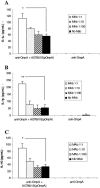
Outer membrane protein A (OmpA) is located in the membrane of Escherichia coli and other gram-negative bacteria and plays a multifunctional role in bacterial physiology and pathogenesis. In enterohemorrhagic E. coli (EHEC), especially serotype O157:H7, OmpA interacts with cultured human intestinal cells and likely acts as an important component to stimulate the immune response during infection. To test this hypothesis, we analyzed the effect of EHEC OmpA on cytokine production by dendritic cells (DCs) and on DC migration across polarized intestinal epithelial cells. OmpA induced murine DCs to secrete interleukin-1 (IL-1), IL-10, and IL-12 in a dose-dependent manner, and this effect was independent of Toll-like receptor 4. Although DCs displayed differential responses to EHEC OmpA and OmpA-specific antibodies enhanced DC cytokine secretion, we cannot discard that other EHEC surface elements were likely to be involved. While OmpA was required for bacterial binding to polarized Caco-2 cells, it was not needed for the induction of cytokine production by Caco-2 cells or for human DC migration across polarized cells.
Figures
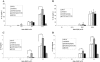

Similar articles
-
Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-κB.Int J Med Microbiol. 2018 Oct;308(7):882-889. doi: 10.1016/j.ijmm.2018.06.004. Epub 2018 Jun 19. Int J Med Microbiol. 2018. PMID: 29934223
-
Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells.Infect Immun. 2003 Sep;71(9):4985-95. doi: 10.1128/IAI.71.9.4985-4995.2003. Infect Immun. 2003. PMID: 12933841 Free PMC article.
-
Development of a Gold Nanoparticle Vaccine against Enterohemorrhagic Escherichia coli O157:H7.mBio. 2019 Aug 13;10(4):e01869-19. doi: 10.1128/mBio.01869-19. mBio. 2019. PMID: 31409688 Free PMC article.
-
Outer membrane protein A expression in Escherichia coli K1 is required to prevent the maturation of myeloid dendritic cells and the induction of IL-10 and TGF-beta.J Immunol. 2008 Aug 15;181(4):2672-82. doi: 10.4049/jimmunol.181.4.2672. J Immunol. 2008. PMID: 18684958
-
In-silico design, expression, and purification of novel chimeric Escherichia coli O157:H7 OmpA fused to LTB protein in Escherichia coli.PLoS One. 2017 Mar 15;12(3):e0173761. doi: 10.1371/journal.pone.0173761. eCollection 2017. PLoS One. 2017. PMID: 28296951 Free PMC article.
Cited by
-
Differential uptake and processing of a Haemophilus influenzae P5-derived immunogen by chinchilla dendritic cells.Infect Immun. 2008 Mar;76(3):967-77. doi: 10.1128/IAI.01395-07. Epub 2007 Dec 26. Infect Immun. 2008. PMID: 18160476 Free PMC article.
-
Specific Proteomic Identification of Collagen-Binding Proteins in Escherichia coli O157:H7: Characterisation of OmpA as a Potent Vaccine Antigen.Cells. 2023 Jun 15;12(12):1634. doi: 10.3390/cells12121634. Cells. 2023. PMID: 37371104 Free PMC article.
-
Outer membrane protein A (OmpA) of Shigella flexneri 2a links innate and adaptive immunity in a TLR2-dependent manner and involvement of IL-12 and nitric oxide.J Biol Chem. 2012 Apr 6;287(15):12589-601. doi: 10.1074/jbc.M111.335554. Epub 2012 Feb 16. J Biol Chem. 2012. Retraction in: J Biol Chem. 2020 Feb 21;295(8):2543. doi: 10.1074/jbc.W120.012819. PMID: 22343631 Free PMC article. Retracted.
-
Protective Immunity Elicited by Oral Immunization of Mice with Salmonella enterica Serovar Typhimurium Braun Lipoprotein (Lpp) and Acetyltransferase (MsbB) Mutants.Front Cell Infect Microbiol. 2016 Nov 10;6:148. doi: 10.3389/fcimb.2016.00148. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27891321 Free PMC article.
-
Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles.Biochim Biophys Acta. 2009 Oct;1788(10):2150-9. doi: 10.1016/j.bbamem.2009.08.001. Epub 2009 Aug 18. Biochim Biophys Acta. 2009. PMID: 19695218 Free PMC article.
References
-
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
-
- Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kappaB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-648. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Caux, C., S. Ait-Yahia, K. Chemin, O. de Bouteiller, M. C. Dieu-Nosjean, B. Homey, C. Massacrier, B. Vanbervliet, A. Zlotnik, and A. Vicari. 2000. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin. Immunopathol. 22:345-369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

