Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine
- PMID: 16622211
- PMCID: PMC1459728
- DOI: 10.1128/IAI.74.5.2742-2750.2006
Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine
Abstract
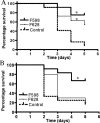
Carbohydrate antigens are important targets of the immune system in clearing bacterial pathogens. Although the immune system almost exclusively uses antibodies in response to foreign carbohydrates, there is still much to learn about the role of different epitopes on the carbohydrate as targets of protective immunity. We examined the role of acetyl group-dependent and -independent epitopes on the staphylococcal surface of polysaccharide poly-N-acetylated glucosamine (PNAG) by use of human monoclonal antibodies (MAbs) specific for such epitopes. We utilized hybridoma technology to produce fully human immunoglobulin G2 (IgG2) MAbs from B cells of an individual post-Staphylococcus aureus infection and cloned the antibody variable regions to produce an IgG1 form of each original MAb. Specificity and functionality of the purified MAbs were tested in vitro using enzyme-linked immunosorbent assays, complement deposition, and opsonophagocytic assays. We found that a MAb (MAb F598) that bound the best to nonacetylated or backbone epitopes on PNAG had superior complement deposition and opsonophagocytic activity compared to two MAbs that bound optimally to PNAG that was expressed with a native level (>90%) of N-acetyl groups (MAbs F628 and F630). Protection of mice against lethality due to S. aureus strains Mn8 and Reynolds further showed that the backbone-specific MAb had optimal protective efficacy compared with the acetate-specific MAbs. These results provide evidence for the importance of epitope specificity in inducing the optimal protective antibody response to PNAG and indicate that MAbs to the deacetylated form of PNAG could be immunotherapeutic agents for preventing or treating staphylococcal infections.
Figures






References
-
- Aase, A., and T. Michaelsen. 1994. Opsonophagocytic activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand. J. Immunol. 39:591-597. - PubMed
-
- Adem, P., C. Montgomery, A. Husain, T. Koogler, A. Arangelovich, M. Humilier, A. Boyle-Vavra, and R. Daum. 2005. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N. Engl. J. Med. 353:1245-1251. - PubMed
-
- Blomster-Hautamaa, D. A., and P. M. Schlievert. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 165:37-43. - PubMed
-
- Boyce, J. 1997. Epidemiology and prevention of nosocomial infections, p. 309-329. In K. Crossley and G. Archer (ed.), The staphylococci in human disease. Churchill Livingston, New York, N.Y.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

