In vivo activation of naive CD4+ T cells in nasal mucosa-associated lymphoid tissue following intranasal immunization with recombinant Streptococcus gordonii
- PMID: 16622213
- PMCID: PMC1459748
- DOI: 10.1128/IAI.74.5.2760-2766.2006
In vivo activation of naive CD4+ T cells in nasal mucosa-associated lymphoid tissue following intranasal immunization with recombinant Streptococcus gordonii
Abstract
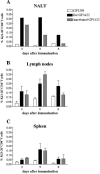
The antigen-specific primary activation of CD4+ T cells was studied in vivo by adoptive transfer of ovalbumin-specific transgenic T cells (KJ1-26+ CD4+) following intranasal immunization with recombinant Streptococcus gordonii. A strain of S. gordonii expressing on its surface a model vaccine antigen fused to the ovalbumin (OVA) peptide from position 323 to 339 was constructed and used to study the OVA-specific T-cell activation in nasal mucosa-associated lymphoid tissue (NALT), lymph nodes, and spleens of mice immunized by the intranasal route. The recombinant strain, but not the wild type, activated the OVA-specific CD4+ T-cell population in the NALT (89% of KJ1-26+ CD4+ T cells) just 3 days following immunization. In the cervical lymph nodes and in the spleen, the percentage of proliferating cells was initially low, but it reached the peak of activation at day 5 (90%). This antigen-specific clonal expansion of KJ1-26+ CD4+ T cells after intranasal immunization was obtained with live and inactivated recombinant bacteria, and it indicates that the NALT is the site of antigen-specific T-cell priming.
Figures



References
-
- Asanuma, H., A. H. Thompson, T. Iwasaki, Y. Sato, Y. Inaba, C. Aizawa, T. Kurata, and S. Tamura. 1997. Isolation and characterization of mouse nasal-associated lymphoid tissue. J. Immunol. Methods 202:123-131. - PubMed
-
- Bienenstock, J., and M. R. McDermott. 2005. Bronchus- and nasal-associated lymphoid tissue. Immunol. Rev. 206:22-31. - PubMed
-
- Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. - PubMed
-
- Corinti, S., D. Medaglini, C. Prezzi, G. Pozzi, and G. Girolomoni. 2000. Human dendritic cells are superior to B cells at presenting a major histocompatibility complex class-II restricted heterologous antigen expressed on recombinant Streptococcus gordonii. Infect. Immun. 68:1879-1883. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

