Protection by meningococcal outer membrane protein PorA-specific antibodies and a serogroup B capsular polysaccharide-specific antibody in complement-sufficient and C6-deficient infant rats
- PMID: 16622217
- PMCID: PMC1459742
- DOI: 10.1128/IAI.74.5.2803-2808.2006
Protection by meningococcal outer membrane protein PorA-specific antibodies and a serogroup B capsular polysaccharide-specific antibody in complement-sufficient and C6-deficient infant rats
Abstract
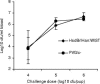
The relative contributions of antibody-induced complement-mediated bacterial lysis and antibody/complement-mediated phagocytosis to host immunity against meningococcal infections are currently unclear. Further, the in vivo effector functions of antibodies may vary depending on their specificity and Fc heavy-chain isotype. In this study, a mouse immunoglobulin G2a (mIgG2a) monoclonal antibody (MN12H2) to meningococcal outer membrane protein PorA (P1.16), its human IgG subclass derivatives (hIgG1 to hIgG4), and an mIgG2a monoclonal antibody (Nmb735) to serogroup B capsular polysaccharide (B-PS) were evaluated for passive protection against meningococcal serogroup B strain 44/76-SL (B:15:P1.7,16) in an infant rat infection model. Complement component C6-deficient (PVG/c-) rats were used to assess the importance of complement-mediated bacterial lysis for protection. The PorA-specific parental mIgG2a and the hIgG1 to hIgG3 derivatives all induced efficient bactericidal activity in vitro in the presence of human or infant rat complement and augmented bacterial clearance in complement-sufficient HsdBrlHan:WIST rats, while the hIgG4 was unable to do so. In C6-deficient PVG/c- rats, lacking complement-mediated bacterial lysis, the augmentation of bacterial clearance by PorA-specific mIgG2a and hIgG1 antibodies was impaired compared to that in the syngeneic complement-sufficient PVG/c+ rat strain. This was in contrast to the case for B-PS-specific mIgG2a, which conferred similar protective activity in both rat strains. These data suggest that while anti-B-PS antibody can provide protection in the infant rats without membrane attack complex formation, the protection afforded by anti-PorA antibody is more dependent on the activation of the whole complement pathway and subsequent bacterial lysis.
Figures



Similar articles
-
Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis.Infect Immun. 2005 Aug;73(8):4694-703. doi: 10.1128/IAI.73.8.4694-4703.2005. Infect Immun. 2005. PMID: 16040982 Free PMC article.
-
Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1.7,16 subtype variants in an infant rat infection model.Microb Pathog. 2001 Mar;30(3):139-48. doi: 10.1006/mpat.2000.0419. Microb Pathog. 2001. PMID: 11273739
-
The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB.Clin Vaccine Immunol. 2006 Apr;13(4):486-91. doi: 10.1128/CVI.13.4.486-491.2006. Clin Vaccine Immunol. 2006. PMID: 16603616 Free PMC article.
-
Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba®).Postgrad Med. 2016 Aug;128(6):548-56. doi: 10.1080/00325481.2016.1203238. Epub 2016 Jul 7. Postgrad Med. 2016. PMID: 27467048 Review.
-
Meningococcal serogroup B vaccines: Estimating breadth of coverage.Hum Vaccin Immunother. 2017 Feb;13(2):255-265. doi: 10.1080/21645515.2017.1264750. Epub 2016 Dec 14. Hum Vaccin Immunother. 2017. PMID: 27960595 Free PMC article. Review.
Cited by
-
Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles.Clin Vaccine Immunol. 2009 Aug;16(8):1113-20. doi: 10.1128/CVI.00118-09. Epub 2009 Jun 24. Clin Vaccine Immunol. 2009. PMID: 19553555 Free PMC article. Clinical Trial.
-
Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease.Vaccine. 2009 Jun 24;27 Suppl 2(Suppl 2):B117-25. doi: 10.1016/j.vaccine.2009.04.066. Epub 2009 May 23. Vaccine. 2009. PMID: 19477054 Free PMC article. Review.
-
Inhibition of the alternative pathway of nonhuman infant complement by porin B2 contributes to virulence of Neisseria meningitidis in the infant rat model.Infect Immun. 2014 Jun;82(6):2574-84. doi: 10.1128/IAI.01517-14. Epub 2014 Mar 31. Infect Immun. 2014. PMID: 24686052 Free PMC article.
-
Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera.Infect Immun. 2009 Feb;77(2):764-9. doi: 10.1128/IAI.01191-08. Epub 2008 Dec 1. Infect Immun. 2009. PMID: 19047406 Free PMC article.
-
Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity.Clin Vaccine Immunol. 2009 Jun;16(6):785-91. doi: 10.1128/CVI.00007-09. Epub 2009 Apr 1. Clin Vaccine Immunol. 2009. PMID: 19339487 Free PMC article.
References
-
- Abdillahi, H., and J. T. Poolman. 1988. Neisseria meningitidis group B serosubtyping using monoclonal antibodies in whole-cell ELISA. Microb. Pathog. 4:27-32. - PubMed
-
- Andreoni, J., H. Käyhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. - PubMed
-
- Figueroa, J., J. Andreoni, and P. Densen. 1993. Complement deficiency states and meningococcal disease. Immunol. Res. 12:295-311. - PubMed
-
- Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Aase, and O. Closs. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

