Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta
- PMID: 16622222
- PMCID: PMC1459757
- DOI: 10.1128/IAI.74.5.2839-2848.2006
Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta
Abstract
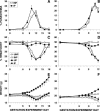
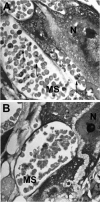
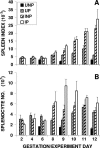
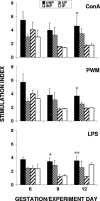
Malarial infection in nonimmune women is a risk factor for pregnancy loss, but the role that maternal antimalarial immune responses play in fetal compromise is not clear. We conducted longitudinal and serial sacrifice studies to examine the pathogenesis of malaria during pregnancy using the Plasmodium chabaudi AS/C57BL/6 mouse model. Peak parasitemia following inoculation with 1,000 parasite-infected murine erythrocytes and survival were similar in infected pregnant and nonpregnant mice, although development of parasitemia and anemia was slightly accelerated in pregnant mice. Importantly, pregnant mice failed to maintain viable pregnancies, most aborting before day 12 of gestation. At abortion, maternal placental blood parasitemia was statistically significantly higher than peripheral parasitemia. Infected mice had similar increases in spleen size and cellularity which were statistically significantly higher than in uninfected mice. In contrast, splenocyte proliferation in response to mitogenic stimulation around peak parasitemia was statistically significantly reduced in both groups of infected mice compared to uninfected, nonpregnant mice, suggesting that lymphoproliferation is not a good indicator of the antimalarial immune responses in pregnant or nonpregnant animals. This study suggests that while pregnant and nonpregnant C57BL/6 mice are equally capable of mounting an effective immune response to and surviving P. chabaudi AS infection, pregnant mice cannot produce viable pups. Fetal loss appears to be associated with placental accumulation of infected erythrocytes. Further study is required to determine to what extent maternal antimalarial immune responses, anemia, and placental accumulation of parasites contribute to compromised pregnancy in this model.
Figures

References
-
- Abdalla, S., and D. J. Weatherall. 1982. The direct antiglobulin test in P. falciparum malaria. Br. J. Haematol. 51:415-425. - PubMed
-
- Abdalla, S., D. J. Weatherall, S. N. Wickramasinghe, and M. Hughes. 1980. The anaemia of P. falciparum malaria. Br. J. Haematol. 46:171-183. - PubMed
-
- Anonymous. 1997. World malaria situation in 1994. Wkly. Epidemiol. Rec. 72:269-276. - PubMed
-
- Beier, J. C., C. N. Oster, F. K. Onyango, J. D. Bales, J. A. Sherwood, P. V. Perkins, D. K. Chumo, D. V. Koech, R. E. Whitmire, C. R. Roberts, et al. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50:529-536. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

