Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study
- PMID: 16622435
- PMCID: PMC2361244
- DOI: 10.1038/sj.bjc.6603075
Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study
Abstract
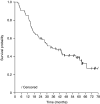
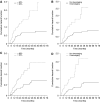
The aim was to investigate the efficacy of neoadjuvant docetaxel-cisplatin and identify prognostic factors for outcome in locally advanced stage IIIA (pN2 by mediastinoscopy) non-small-cell lung cancer (NSCLC) patients. In all, 75 patients (from 90 enrolled) underwent tumour resection after three 3-week cycles of docetaxel 85 mg m-2 (day 1) plus cisplatin 40 or 50 mg m-2 (days 1 and 2). Therapy was well tolerated (overall grade 3 toxicity occurred in 48% patients; no grade 4 nonhaematological toxicity was reported), with no observed late toxicities. Median overall survival (OS) and event-free survival (EFS) times were 35 and 15 months, respectively, in the 75 patients who underwent surgery; corresponding figures for all 90 patients enrolled were 28 and 12 months. At 3 years after initiating trial therapy, 27 out of 75 patients (36%) were alive and tumour free. At 5-year follow-up, 60 and 65% of patients had local relapse and distant metastases, respectively. The most common sites of distant metastases were the lung (24%) and brain (17%). Factors associated with OS, EFS and risk of local relapse and distant metastases were complete tumour resection and chemotherapy activity (clinical response, pathologic response, mediastinal downstaging). Neoadjuvant docetaxel-cisplatin was effective and tolerable in stage IIIA pN2 NSCLC, with chemotherapy contributing significantly to outcomes.
Figures
References
-
- Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi III AT, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR (1995) Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 13: 1880–1892 - PubMed
-
- Albain KS, Scott CB, Rusch VR, Turrisi AT, Shepherd SA, Smith C, Gandara DR, Johnson D, Green MR, Miller RC (2003) Phase III study of concurrent chemotherapy and full course radiotherapy (CR/RT) versus CT/RT induction followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer (NSCLC): first outcome analysis of North American Intergroup trial 0139 (RTOG 93-09). Lung Cancer 41(Suppl 2): S4 PL-4
-
- Albain KS, Swann RS, Rusch VR, Turrisi AT, Shepherd FA, Smith CJ, Gandara DR, Johnson DH, Green MR, Miller RC, the North American Lung Cancer Intergroup (2005) Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer (NSCLC): outcomes update of North American Intergroup 0139 (RTOG 9309). J Clin Oncol 23(Suppl 16S): 624s (Abstr 7014)
-
- Andre F, Grunenwald D, Pujol JL, Girard P, Dujon A, Brouchet L, Brichon PY, Westeel V, Le Chevalier T (2001) Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: should prophylactic cranial irradiation be reconsidered? Cancer 91: 2394–2400 - PubMed
-
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, the International Adjuvant Lung Cancer Trial Collaborative Group (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350: 351–360 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

