The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines
- PMID: 16622460
- PMCID: PMC2361410
- DOI: 10.1038/sj.bjc.6603088
The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines
Abstract
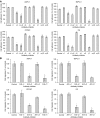
Pancreatic cancer is characterised by a hallmark desmoplastic response that includes upregulated expression of the extracellular matrix, and type I collagen in particular. Recent studies indicate that pancreatic cancer cells stimulate type I collagen synthesis in adjacent stellate cells, and that this upregulated type I collagen expression promotes the malignant phenotype in tumour cells as defined by increased proliferation, resistance to chemically induced apoptosis, and increased tumorigenesis. The integrin specificity of this interaction between type I collagen and tumour cells was not identified, however. In the present study, we examined eight pancreatic cancer cell lines for adhesion, proliferation, and migration, on types I and IV collagen, fibronectin, laminin, and vitronectin, as well as integrin expression. Our results indicate, for the overwhelming majority of cell lines, that type I collagen promotes the strongest adhesion, proliferation, and migration relative to the other substrates tested. Utilising function-blocking monoclonal antibodies directed against particular integrin subunits in cell adhesion and migration inhibition assays, we demonstrate further that the malignant phenotype on type I collagen is mediated specifically by the alpha2beta1 integrin. These results identify alpha2beta1 integrin-mediated adhesion to type I collagen as a potential therapeutic target in the treatment of pancreatic cancer.
Figures




References
-
- Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP (2004) Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res 10: 7427–7437 - PubMed
-
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128: 907–921 - PubMed
-
- Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2: 897–909 - PubMed
-
- Binkley CE, Zhang L, Greenson JK, Giordano TJ, Kuick R, Misek D, Hanash S, Logsdon CD, Simeone DM (2004) The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas 29: 254–263 - PubMed
-
- Cheresh DA, Spiro RC (1987) Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem 262: 17703–17711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

