Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy
- PMID: 16623949
- PMCID: PMC1462997
- DOI: 10.1186/1742-4690-3-24
Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy
Abstract
Background: Many novel studies and therapies are possible with the use of human embryonic stem cells (hES cells) and their differentiated cell progeny. The hES cell derived CD34 hematopoietic stem cells can be potentially used for many gene therapy applications. Here we evaluated the capacity of hES cell derived CD34 cells to give rise to normal macrophages as a first step towards using these cells in viral infection studies and in developing novel stem cell based gene therapy strategies for AIDS.
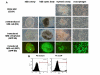
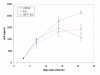
Results: Undifferentiated normal and lentiviral vector transduced hES cells were cultured on S17 mouse bone marrow stromal cell layers to derive CD34 hematopoietic progenitor cells. The differentiated CD34 cells isolated from cystic bodies were further cultured in cytokine media to derive macrophages. Phenotypic and functional analyses were carried out to compare these with that of fetal liver CD34 cell derived macrophages. As assessed by FACS analysis, the hES-CD34 cell derived macrophages displayed characteristic cell surface markers CD14, CD4, CCR5, CXCR4, and HLA-DR suggesting a normal phenotype. Tests evaluating phagocytosis, upregulation of the costimulatory molecule B7.1, and cytokine secretion in response to LPS stimulation showed that these macrophages are also functionally normal. When infected with HIV-1, the differentiated macrophages supported productive viral infection. Lentiviral vector transduced hES cells expressing the transgene GFP were evaluated similarly like above. The transgenic hES cells also gave rise to macrophages with normal phenotypic and functional characteristics indicating no vector mediated adverse effects during differentiation.
Conclusion: Phenotypically normal and functionally competent macrophages could be derived from hES-CD34 cells. Since these cells are susceptible to HIV-1 infection, they provide a uniform source of macrophages for viral infection studies. Based on these results, it is also now feasible to transduce hES-CD34 cells with anti-HIV genes such as inhibitory siRNAs and test their antiviral efficacy in down stream differentiated cells such as macrophages which are among the primary cells that need to be protected against HIV-1 infection. Thus, the potential utility of hES derived CD34 hematopoietic cells for HIV-1 gene therapy can be evaluated.
Figures


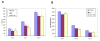
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

